SHIV-C109p5 NHP induces rapid disease progression in elderly macaques with extensive GI viral replication
- PMID: 38299866
- PMCID: PMC10878093
- DOI: 10.1128/jvi.01652-23
SHIV-C109p5 NHP induces rapid disease progression in elderly macaques with extensive GI viral replication
Abstract
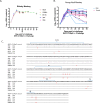
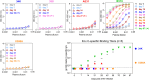
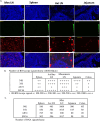
CCR5-tropic simian/human immunodeficiency viruses (SHIV) with clade C transmitted/founder envelopes represent a critical tool for the investigation of HIV experimental vaccines and microbicides in nonhuman primates, although many such isolates lead to spontaneous viral control post infection. Here, we generated a high-titer stock of pathogenic SHIV-C109p5 by serial passage in two rhesus macaques (RM) and tested its virulence in aged monkeys. The co-receptor usage was confirmed before infecting five geriatric rhesus macaques (four female and one male). Plasma viral loads were monitored by reverse transcriptase-quantitative PCR (RT-qPCR), cytokines by multiplex analysis, and biomarkers of gastrointestinal damage by enzyme-linked immunosorbent assay. Antibodies and cell-mediated responses were also measured. Viral dissemination into tissues was determined by RNAscope. Intravenous SHIV-C109p5 infection of aged RMs leads to high plasma viremia and rapid disease progression; rapid decrease in CD4+ T cells, CD4+CD8+ T cells, and plasmacytoid dendritic cells; and wasting necessitating euthanasia between 3 and 12 weeks post infection. Virus-specific cellular immune responses were detected only in the two monkeys that survived 4 weeks post infection. These were Gag-specific TNFα+CD8+, MIP1β+CD4+, Env-specific IFN-γ+CD4+, and CD107a+ T cell responses. Four out of five monkeys had elevated intestinal fatty acid binding protein levels at the viral peak, while regenerating islet-derived protein 3α showed marked increases at later time points in the three animals surviving the longest, suggesting gut antimicrobial peptide production in response to microbial translocation post infection. Plasma levels of monocyte chemoattractant protein-1, interleukin-15, and interleukin-12/23 were also elevated. Viral replication in gut and secondary lymphoid tissues was extensive.IMPORTANCESimian/human immunodeficiency viruses (SHIV) are important reagents to study prevention of virus acquisition in nonhuman primate models of HIV infection, especially those representing transmitted/founder (T/F) viruses. However, many R5-tropic SHIV have limited fitness in vivo leading to many monkeys spontaneously controlling the virus post acute infection. Here, we report the generation of a pathogenic SHIV clade C T/F stock by in vivo passage leading to sustained viral load set points, a necessity to study pathogenicity. Unexpectedly, administration of this SHIV to elderly rhesus macaques led to extensive viral replication and fast disease progression, despite maintenance of a strict R5 tropism. Such age-dependent rapid disease progression had previously been reported for simian immunodeficiency virus but not for R5-tropic SHIV infections.
Keywords: AIDS; SHIV; aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Novitsky V, Smith UR, Gilbert P, McLane MF, Chigwedere P, Williamson C, Ndung’u T, Klein I, Chang SY, Peter T, Thior I, Foley BT, Gaolekwe S, Rybak N, Gaseitsiwe S, Vannberg F, Marlink R, Lee TH, Essex M. 2002. Human immunodeficiency virus type 1 subtype C molecular phylogeny: consensus sequence for an AIDS vaccine design? J Virol 76:5435–5451. doi:10.1128/JVI.76.11.5435-5451.2002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

