Cellular arrangement impacts metabolic activity and antibiotic tolerance in Pseudomonas aeruginosa biofilms
- PMID: 38300958
- PMCID: PMC10833521
- DOI: 10.1371/journal.pbio.3002205
Cellular arrangement impacts metabolic activity and antibiotic tolerance in Pseudomonas aeruginosa biofilms
Abstract
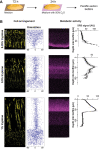
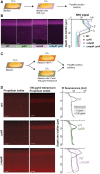
Cells must access resources to survive, and the anatomy of multicellular structures influences this access. In diverse multicellular eukaryotes, resources are provided by internal conduits that allow substances to travel more readily through tissue than they would via diffusion. Microbes growing in multicellular structures, called biofilms, are also affected by differential access to resources and we hypothesized that this is influenced by the physical arrangement of the cells. In this study, we examined the microanatomy of biofilms formed by the pathogenic bacterium Pseudomonas aeruginosa and discovered that clonal cells form striations that are packed lengthwise across most of a mature biofilm's depth. We identified mutants, including those defective in pilus function and in O-antigen attachment, that show alterations to this lengthwise packing phenotype. Consistent with the notion that cellular arrangement affects access to resources within the biofilm, we found that while the wild type shows even distribution of tested substrates across depth, the mutants show accumulation of substrates at the biofilm boundaries. Furthermore, we found that altered cellular arrangement within biofilms affects the localization of metabolic activity, the survival of resident cells, and the susceptibility of subpopulations to antibiotic treatment. Our observations provide insight into cellular features that determine biofilm microanatomy, with consequences for physiological differentiation and drug sensitivity.
Copyright: © 2024 Dayton et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Update of
-
Cell arrangement impacts metabolic activity and antibiotic tolerance in Pseudomonas aeruginosa biofilms.bioRxiv [Preprint]. 2023 Aug 28:2023.06.20.545666. doi: 10.1101/2023.06.20.545666. bioRxiv. 2023. Update in: PLoS Biol. 2024 Feb 1;22(2):e3002205. doi: 10.1371/journal.pbio.3002205. PMID: 37645902 Free PMC article. Updated. Preprint.
References
-
- Mah TF O ’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

