Neutralizing monoclonal antibodies against the Gc fusion loop region of Crimean-Congo hemorrhagic fever virus
- PMID: 38300972
- PMCID: PMC10863865
- DOI: 10.1371/journal.ppat.1011948
Neutralizing monoclonal antibodies against the Gc fusion loop region of Crimean-Congo hemorrhagic fever virus
Abstract
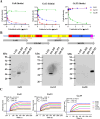
Crimean-Congo hemorrhagic fever virus (CCHFV) is a highly pathogenic tick-borne virus, prevalent in more than 30 countries worldwide. Human infection by this virus leads to severe illness, with an average case fatality of 40%. There is currently no approved vaccine or drug to treat the disease. Neutralizing antibodies are a promising approach to treat virus infectious diseases. This study generated 37 mouse-derived specific monoclonal antibodies against CCHFV Gc subunit. Neutralization assays using pseudotyped virus and authentic CCHFV identified Gc8, Gc13, and Gc35 as neutralizing antibodies. Among them, Gc13 had the highest neutralizing activity and binding affinity with CCHFV Gc. Consistently, Gc13, but not Gc8 or Gc35, showed in vivo protective efficacy (62.5% survival rate) against CCHFV infection in a lethal mouse infection model. Further characterization studies suggested that Gc8 and Gc13 may recognize a similar, linear epitope in domain II of CCHFV Gc, while Gc35 may recognize a different epitope in Gc. Cryo-electron microscopy of Gc-Fab complexes indicated that both Gc8 and Gc13 bind to the conserved fusion loop region and Gc13 had stronger interactions with sGc-trimers. This was supported by the ability of Gc13 to block CCHFV GP-mediated membrane fusion. Overall, this study provides new therapeutic strategies to treat CCHF and new insights into the interaction between antibodies with CCHFV Gc proteins.
Copyright: © 2024 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
M.W., L.L., Z.H., S.C., L.P., and Y.S. filed a patent for Gc13 in treatment of CCHF to China Intellectual Property Office. Remaining authors declare no conflicts of interests.
Figures
Similar articles
-
Avidity and variable domain spacing strongly influence the therapeutic potency of bispecific antibodies against Crimean-Congo hemorrhagic fever virus.mBio. 2025 May 14;16(5):e0320224. doi: 10.1128/mbio.03202-24. Epub 2025 Apr 16. mBio. 2025. PMID: 40237506 Free PMC article.
-
Immunization with DNA Plasmids Coding for Crimean-Congo Hemorrhagic Fever Virus Capsid and Envelope Proteins and/or Virus-Like Particles Induces Protection and Survival in Challenged Mice.J Virol. 2017 Apr 28;91(10):e02076-16. doi: 10.1128/JVI.02076-16. Print 2017 May 15. J Virol. 2017. PMID: 28250124 Free PMC article.
-
Nucleoside-Modified mRNA Vaccines Protect IFNAR-/- Mice against Crimean-Congo Hemorrhagic Fever Virus Infection.J Virol. 2022 Feb 9;96(3):e0156821. doi: 10.1128/JVI.01568-21. Epub 2021 Nov 24. J Virol. 2022. PMID: 34817199 Free PMC article.
-
Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity.Antiviral Res. 2013 Oct;100(1):159-89. doi: 10.1016/j.antiviral.2013.07.006. Epub 2013 Jul 29. Antiviral Res. 2013. PMID: 23906741 Review.
-
Crimean-Congo Hemorrhagic Fever.Lab Med. 2015 Summer;46(3):180-9. doi: 10.1309/LMN1P2FRZ7BKZSCO. Lab Med. 2015. PMID: 26199256 Review.
Cited by
-
Mucin-like protein of Crimean-Congo hemorrhagic fever virus is a key virulence factor and a potent target for developing novel attenuated vaccine.Cell Res. 2025 Jul;35(7):524-527. doi: 10.1038/s41422-025-01130-7. Epub 2025 May 16. Cell Res. 2025. PMID: 40374949 No abstract available.
-
The emergence of oropouche fever: A potential new threat?New Microbes New Infect. 2025 May 10;65:101596. doi: 10.1016/j.nmni.2025.101596. eCollection 2025 Jun. New Microbes New Infect. 2025. PMID: 40491501 Free PMC article.
-
Viral hijacking of host DDX60 promotes Crimean-Congo haemorrhagic fever virus replication via G-quadruplex unwinding.PLoS Pathog. 2025 Jun 27;21(6):e1013278. doi: 10.1371/journal.ppat.1013278. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40577441 Free PMC article.
-
Recent Advances in Crimean-Congo Hemorrhagic Fever Virus Detection, Treatment, and Vaccination: Overview of Current Status and Challenges.Biol Proced Online. 2024 Jun 26;26(1):20. doi: 10.1186/s12575-024-00244-3. Biol Proced Online. 2024. PMID: 38926669 Free PMC article. Review.
-
Avidity and variable domain spacing strongly influence the therapeutic potency of bispecific antibodies against Crimean-Congo hemorrhagic fever virus.mBio. 2025 May 14;16(5):e0320224. doi: 10.1128/mbio.03202-24. Epub 2025 Apr 16. mBio. 2025. PMID: 40237506 Free PMC article.
References
-
- Oestereich L, Rieger T, Neumann M, Bernreuther C, Lehmann M, Krasemann S, et al.. Evaluation of Antiviral Efficacy of Ribavirin, Arbidol, and T-705 (Favipiravir) in a Mouse Model for Crimean-Congo Hemorrhagic Fever. PLoS Negl Trop Dis. 2014;8:e2804. doi: 10.1371/journal.pntd.0002804 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

