Mechanism of Ventricular Tachycardia Occurring in Chronic Myocardial Infarction Scar
- PMID: 38300981
- PMCID: PMC10836816
- DOI: 10.1161/CIRCRESAHA.123.321553
Mechanism of Ventricular Tachycardia Occurring in Chronic Myocardial Infarction Scar
Abstract
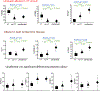
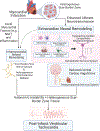
Cardiac arrest is the leading cause of death in the more economically developed countries. Ventricular tachycardia associated with myocardial infarct is a prominent cause of cardiac arrest. Ventricular arrhythmias occur in 3 phases of infarction: during the ischemic event, during the healing phase, and after the scar matures. Mechanisms of arrhythmias in these phases are distinct. This review focuses on arrhythmia mechanisms for ventricular tachycardia in mature myocardial scar. Available data have shown that postinfarct ventricular tachycardia is a reentrant arrhythmia occurring in circuits found in the surviving myocardial strands that traverse the scar. Electrical conduction follows a zigzag course through that area. Conduction velocity is impaired by decreased gap junction density and impaired myocyte excitability. Enhanced sympathetic tone decreases action potential duration and increases sarcoplasmic reticular calcium leak and triggered activity. These elements of the ventricular tachycardia mechanism are found diffusely throughout scar. A distinct myocyte repolarization pattern is unique to the ventricular tachycardia circuit, setting up conditions for classical reentry. Our understanding of ventricular tachycardia mechanisms continues to evolve as new data become available. The ultimate use of this information would be the development of novel diagnostics and therapeutics to reliably identify at-risk patients and prevent their ventricular arrhythmias.
Keywords: cause of death; chronic total occlusion; complications; death; mortality.
Conflict of interest statement
Figures



References
-
- Bridges C, Burkman J, Malekan R, Konig S, Chen H, Yarnall C, Gardner T, Stewart A, Stecker M, Patterson T and Stedman H. Global cardiac-specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann Thorac Surg. 2002;73:1939–1946. - PubMed
-
- Berdowski J, Berg RA, Tijssen JG and Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–87. - PubMed
-
- Pouleur AC, Barkoudah E, Uno H, Skali H, Finn PV, Zelenkofske SL, Belenkov YN, Mareev V, Velazquez EJ, Rouleau JL, Maggioni AP, Kober L, Califf RM, McMurray JJ, Pfeffer MA and Solomon SD. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122:597–602. - PubMed
-
- Janse MJ and Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

