Addition of Carboxylic Acids to gem-Difluoroalkenes for the Synthesis of gem-Difluoromethylenated Compounds
- PMID: 38301042
- PMCID: PMC10877607
- DOI: 10.1021/acs.orglett.4c00095
Addition of Carboxylic Acids to gem-Difluoroalkenes for the Synthesis of gem-Difluoromethylenated Compounds
Abstract
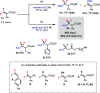
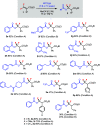
We herein describe a straightforward protocol for the synthesis of carboxylic esters containing a gem-difluoromethylene unit. Readily available carboxylic acids can act as nucleophiles to add regioselectively to tetrasubstituted or trisubstituted β,β-difluoroacrylates (formal hydroacetoxylation) for the construction of RCO2-CF2 bonds. Thermal conditions are sufficient without the use of catalysts or additives.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Krishnamoorthy S.; Prakash G. K. S. Silicon-Based Reagents for Difluoromethylation and Difluoromethylenation Reactions. Synthesis 2017, 49, 3394–3406. 10.1055/s-0036-1588489. - DOI
- Yerien D. E.; Barata-Vallejo S.; Postigo A. Difluoromethylation Reactions of Organic Compounds. Chem. - Eur. J. 2017, 23, 14676–14701. 10.1002/chem.201702311. - DOI - PubMed
- Sap J. B. I.; Meyer C. F.; Straathof N. J. W.; Iwumene N.; am Ende C. W.; Trabanco A. S.; Gouverneur V. Late-Stage Difluoromethylation: Concepts, Developments and Perspective. Chem. Soc. Rev. 2021, 50, 8214–8247. 10.1039/D1CS00360G. - DOI - PubMed
-
- Holovach S.; Melnykov K. P.; Skreminskiy A.; Herasymchuk M.; Tavlui O.; Aloshyn D.; Borysko P.; Rozhenko A. B.; Ryabukhin S. V.; Volochnyuk D. M.; Grygorenko O. O. Effect of gem-Difluorination on the Key Physicochemical Properties Relevant to Medicinal Chemistry: The Case of Functionalized Cycloalkanes. Chem. - Eur. J. 2022, 28, e20220033 10.1002/chem.202200331. - DOI - PubMed
-
- Sorrentino J. P.; Altman R. A. Fluorine-Retentive Strategies for the Functionalization of gem-Difluoroalkenes. Synthesis 2021, 53, 3935–3950. 10.1055/a-1547-9270. - DOI - PMC - PubMed
- Liu C.; Zeng H.; Zhu C.; Jiang H. Recent Advances in Three-Component Difunctionalization of gem-Difluoroalkenes. Chem. Commun. 2020, 56, 10442–10452. 10.1039/D0CC04318D. - DOI - PubMed
- Nguyen B. V.; Burton D. J. A New Route for the Preparation of Substituted 2,2-Difluorostyrenes and A Convenient Route to Substituted (2,2,2-Trifluoroethyl)benzenes. J. Org. Chem. 1997, 62, 7758–7764. 10.1021/jo971019w. - DOI
- Riss P. J.; Aigbirhio F. I. A simple, Rapid Procedure for Nucleophilic Radiosynthesis of Aliphatic [18F]Trifluoromethyl Groups. Chem. Commun. 2011, 47, 11873–11875. 10.1039/c1cc15342k. - DOI - PubMed
-
- Fujita T.; Fuchibe K.; Ichikawa J. Transition-Metal-Mediated and -Catalyzed C-F Bond Activation by Fluorine Elimination. Angew. Chem., Int. Ed. 2019, 58, 390–402. 10.1002/anie.201805292. - DOI - PubMed
- Zhang X.; Cao S. Recent Advances in the Synthesis and C-F Functionalization of gem-Difluoroalkenes. Tetrahedron Lett. 2017, 58, 375–392. 10.1016/j.tetlet.2016.12.054. - DOI
- Amii H.; Uneyama K. C-F. Bond Activation in Organic Synthesis. Chem. Rev. 2009, 109, 2119–2183. 10.1021/cr800388c. - DOI - PubMed
-
- Orsi D. L.; Easley B. J.; Lick A. M.; Altman R. A. Base Catalysis Enables Access to α. α-Difluoroalkylthioethers. Org. Lett. 2017, 19, 1570–1573. 10.1021/acs.orglett.7b00386. - DOI - PMC - PubMed
- Orsi D. L.; Yadav M. R.; Altman R. A. Organocatalytic Strategy for Hydrophenolation of gem-Difluoroalkenes. Tetrahedron 2019, 75, 4325–4336. 10.1016/j.tet.2019.04.016. - DOI - PMC - PubMed
- Sorrentino J. P.; Orsi D. L.; Altman R. A. Acid-Catalyzed Hydrothiolation of gem-Difluorostyrenes to Access α,α-Difluoroalkylthioethers. J. Org. Chem. 2021, 86, 2297–2311. 10.1021/acs.joc.0c02440. - DOI - PMC - PubMed
- Herrick R. M.; Abd El-Gaber M. K.; Coy G.; Altman R. A. A Diselenide Additive Enables Photocatalytic Hydroalkoxylation of gem-Difluoroalkenes. Chem. Commun. 2023, 59, 5623–5626. 10.1039/D3CC01012K. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

