Lung Epithelium Releases Growth Differentiation Factor 15 in Response to Pathogen-mediated Injury
- PMID: 38301257
- PMCID: PMC11109583
- DOI: 10.1165/rcmb.2023-0429OC
Lung Epithelium Releases Growth Differentiation Factor 15 in Response to Pathogen-mediated Injury
Abstract
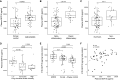
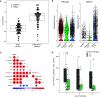
GDF15 (growth differentiation factor 15) is a stress cytokine with several proposed roles, including support of stress erythropoiesis. Higher circulating GDF15 levels are prognostic of mortality during acute respiratory distress syndrome, but the cellular sources and downstream effects of GDF15 during pathogen-mediated lung injury are unclear. We quantified GDF15 in lower respiratory tract biospecimens and plasma from patients with acute respiratory failure. Publicly available data from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were reanalyzed. We used mouse models of hemorrhagic acute lung injury mediated by Pseudomonas aeruginosa exoproducts in wild-type mice and mice genetically deficient for Gdf15 or its putative receptor, Gfral. In critically ill humans, plasma levels of GDF15 correlated with lower respiratory tract levels and were higher in nonsurvivors. SARS-CoV-2 infection induced GDF15 expression in human lung epithelium, and lower respiratory tract GDF15 levels were higher in coronavirus disease (COVID-19) nonsurvivors. In mice, intratracheal P. aeruginosa type II secretion system exoproducts were sufficient to induce airspace and plasma release of GDF15, which was attenuated with epithelial-specific deletion of Gdf15. Mice with global Gdf15 deficiency had decreased airspace hemorrhage, an attenuated cytokine profile, and an altered lung transcriptional profile during injury induced by P. aeruginosa type II secretion system exoproducts, which was not recapitulated in mice deficient for Gfral. Airspace GDF15 reconstitution did not significantly modulate key lung cytokine levels but increased circulating erythrocyte counts. Lung epithelium releases GDF15 during pathogen injury, which is associated with plasma levels in humans and mice and can increase erythrocyte counts in mice, suggesting a novel lung-blood communication pathway.
Keywords: GDF15; GFRAL; acute respiratory failure; cytokines; stress erythropoiesis.
Figures





References
-
- Mullican SE, Lin-Schmidt X, Chin C-N, Chavez JA, Furman JL, Armstrong AA, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med . 2017;23:1150–1157. - PubMed
-
- Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med . 2017;23:1215–1219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- IK2 BX004886/BX/BLRD VA/United States
- R38 HL150207/HL/NHLBI NIH HHS/United States
- R01 AA028436/AA/NIAAA NIH HHS/United States
- K24 HL143285/HL/NHLBI NIH HHS/United States
- R35 HL161177/HL/NHLBI NIH HHS/United States
- R01 HL136143/HL/NHLBI NIH HHS/United States
- R01 HL142084/HL/NHLBI NIH HHS/United States
- P01 HL114453/HL/NHLBI NIH HHS/United States
- R01 HL146519/HL/NHLBI NIH HHS/United States
- K08 HL163324/HL/NHLBI NIH HHS/United States
- R01 HL159805/HL/NHLBI NIH HHS/United States
- T32 HL007563/HL/NHLBI NIH HHS/United States
- R03 HL162655/HL/NHLBI NIH HHS/United States
- K23 GM122069/GM/NIGMS NIH HHS/United States
- K23 GM122069/HL/NHLBI NIH HHS/United States
- R01 HL135062/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

