Brain cholesterol and Alzheimer's disease: challenges and opportunities in probe and drug development
- PMID: 38301270
- PMCID: PMC11068113
- DOI: 10.1093/brain/awae028
Brain cholesterol and Alzheimer's disease: challenges and opportunities in probe and drug development
Abstract
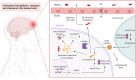
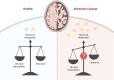
Cholesterol homeostasis is impaired in Alzheimer's disease; however, attempts to modulate brain cholesterol biology have not translated into tangible clinical benefits for patients to date. Several recent milestone developments have substantially improved our understanding of how excess neuronal cholesterol contributes to the pathophysiology of Alzheimer's disease. Indeed, neuronal cholesterol was linked to the formation of amyloid-β and neurofibrillary tangles through molecular pathways that were recently delineated in mechanistic studies. Furthermore, remarkable advances in translational molecular imaging have now made it possible to probe cholesterol metabolism in the living human brain with PET, which is an important prerequisite for future clinical trials that target the brain cholesterol machinery in Alzheimer's disease patients-with the ultimate aim being to develop disease-modifying treatments. This work summarizes current concepts of how the biosynthesis, transport and clearance of brain cholesterol are affected in Alzheimer's disease. Further, current strategies to reverse these alterations by pharmacotherapy are critically discussed in the wake of emerging translational research tools that support the assessment of brain cholesterol biology not only in animal models but also in patients with Alzheimer's disease.
Keywords: Alzheimer’s disease; brain cholesterol homeostasis; cholesterol lowering therapy; cytochrome P450 46A1 (CYP46A1); translational molecular imaging.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
S.H.L. and A.H. are listed as inventors on the provisional patent application ‘Novel PET ligands for imaging cholesterol homeostasis’ (application number 63/397,463).
Figures

References
-
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. - PubMed
-
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17:327–406. - PubMed
-
- Alzheimer’s Association . 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168. - PubMed
-
- Selkoe DJ. Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Small GW, Kepe V, Ercoli LM, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–2663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

