IL-23 induces CLEC5A+ IL-17A+ neutrophils and elicit skin inflammation associated with psoriatic arthritis
- PMID: 38301504
- PMCID: PMC10981569
- DOI: 10.1016/j.jaut.2024.103167
IL-23 induces CLEC5A+ IL-17A+ neutrophils and elicit skin inflammation associated with psoriatic arthritis
Abstract
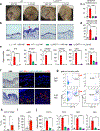
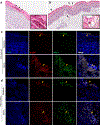
IL-23-activation of IL-17 producing T cells is involved in many rheumatic diseases. Herein, we investigate the role of IL-23 in the activation of myeloid cell subsets that contribute to skin inflammation in mice and man. IL-23 gene transfer in WT, IL-23RGFP reporter mice and subsequent analysis with spectral cytometry show that IL-23 regulates early innate immune events by inducing the expansion of a myeloid MDL1+CD11b+Ly6G+ population that dictates epidermal hyperplasia, acanthosis, and parakeratosis; hallmark pathologic features of psoriasis. Genetic ablation of MDL-1, a major PU.1 transcriptional target during myeloid differentiation exclusively expressed in myeloid cells, completely prevents IL-23-pathology. Moreover, we show that IL-23-induced myeloid subsets are also capable of producing IL-17A and IL-23R+MDL1+ cells are present in the involved skin of psoriasis patients and gene expression correlations between IL-23 and MDL-1 have been validated in multiple patient cohorts. Collectively, our data demonstrate a novel role of IL-23 in MDL-1-myelopoiesis that is responsible for skin inflammation and related pathologies. Our data open a new avenue of investigations regarding the role of IL-23 in the activation of myeloid immunoreceptors and their role in autoimmunity.
Keywords: Arthritis; Autoimmune diseases; Autoimmunity; Inflammation; Psoriatic.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts of interest to declare.
Figures





Similar articles
-
IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis.Ann Rheum Dis. 2015 Jun;74(6):1284-92. doi: 10.1136/annrheumdis-2013-204782. Epub 2014 Feb 23. Ann Rheum Dis. 2015. PMID: 24567524 Free PMC article.
-
Differential Requirement for CCR6 in IL-23-Mediated Skin and Joint Inflammation.J Invest Dermatol. 2020 Dec;140(12):2386-2397. doi: 10.1016/j.jid.2020.03.965. Epub 2020 Apr 24. J Invest Dermatol. 2020. PMID: 32339538
-
Reactive oxygen species produced by myeloid cells in psoriasis as a potential biofactor contributing to the development of vascular inflammation.Biofactors. 2023 Jul-Aug;49(4):861-874. doi: 10.1002/biof.1949. Epub 2023 May 4. Biofactors. 2023. PMID: 37139784
-
Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis.Immunology. 2014 Feb;141(2):133-42. doi: 10.1111/imm.12142. Immunology. 2014. PMID: 23819583 Free PMC article. Review.
-
The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis.Clin Rev Allergy Immunol. 2018 Dec;55(3):379-390. doi: 10.1007/s12016-018-8702-3. Clin Rev Allergy Immunol. 2018. PMID: 30109481 Free PMC article. Review.
Cited by
-
Cardiometabolic Risk in Psoriatic Arthritis: A Hidden Burden of Inflammation and Metabolic Dysregulation.Metabolites. 2025 Mar 18;15(3):206. doi: 10.3390/metabo15030206. Metabolites. 2025. PMID: 40137170 Free PMC article. Review.
-
Pathogenesis of psoriatic arthritis: new insights from a bone marrow perspective.Curr Opin Rheumatol. 2025 Mar 1;37(2):136-141. doi: 10.1097/BOR.0000000000001064. Epub 2024 Oct 29. Curr Opin Rheumatol. 2025. PMID: 39470182 Review.
-
Psoriatic arthritis: diagnosis, pathogenesis, and emerging therapies.Trends Mol Med. 2025 Jul;31(7):682-683. doi: 10.1016/j.molmed.2025.05.003. Epub 2025 Jun 10. Trends Mol Med. 2025. PMID: 40500630 No abstract available.
References
-
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity, 2009;31:331–41. - PubMed
-
- Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P et al. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol, 2007;179:8274–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

