Impact of HIV self-testing for oral pre-exposure prophylaxis scale-up on drug resistance and HIV outcomes in western Kenya: a modelling study
- PMID: 38301668
- PMCID: PMC10896737
- DOI: 10.1016/S2352-3018(23)00268-0
Impact of HIV self-testing for oral pre-exposure prophylaxis scale-up on drug resistance and HIV outcomes in western Kenya: a modelling study
Abstract
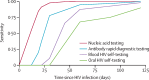
Background: Community-based oral pre-exposure prophylaxis (PrEP) provision has the potential to expand PrEP coverage. HIV self-testing can facilitate PrEP community-based delivery but might have lower sensitivity than facility-based HIV testing, potentially leading to inappropriate PrEP use among people with HIV and subsequent development of drug resistance. We aimed to evaluate the impact of HIV self-testing use for PrEP scale-up.
Methods: We parameterised an agent-based network model, EMOD-HIV, to simulate generic tenofovir disoproxil fumarate and emtricitabine PrEP scale-up in western Kenya using four testing scenarios: provider-administered nucleic acid testing, provider-administered rapid diagnostic tests detecting antibodies, blood-based HIV self-testing, or oral fluid HIV self-testing. Scenarios were compared with a no PrEP counterfactual. Individuals aged 18-49 years with one or more heterosexual partners who screened HIV-negative were eligible for PrEP. We assessed the cost and health impact of rapid PrEP scale-up with high coverage over 20 years, and the budget impact over 5 years, using various HIV testing modalities.
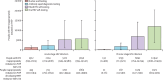
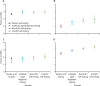
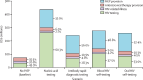
Findings: PrEP coverage of 29% was projected to avert approximately 54% of HIV infections and 17% of HIV-related deaths among adults aged 18-49 years over 20 years; health impacts were similar across HIV testing modalities used to deliver PrEP. The percentage of HIV infections with PrEP-associated nucleoside reverse transcriptase inhibitor (NRTI) drug resistance was 0·6% (95% uncertainty intervals 0·4-0·9) in the blood HIV self-testing scenario and 0·8% (0·6-1·0) in the oral HIV self-testing scenario, compared with 0·3% (0·2-0·3) in the antibody rapid diagnostic testing scenario and 0·2% (0·1-0·2) in the nucleic acid testing scenario. Accounting for background NRTI resistance, we found similarly low proportions of drug resistance across scenarios. The budget impact of implementing PrEP using HIV self-testing and provider-administered rapid diagnostic tests were similar, while nucleic acid testing was approximately 50% more costly.
Interpretation: Scaling up PrEP using HIV self-testing has similar health impacts, costs, and low risk of drug resistance as provider-administered rapid diagnostic tests. Policy makers should consider leveraging HIV self-testing to expand PrEP access among those at HIV risk.
Funding: The Bill and Melinda Gates Foundation.
© 2024 World Health Organization. Published by Elsevier Ltd. All rights reserved. This is an Open Access article published under the CC BY 3.0 IGO license which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any use of this article, there should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Conflict of interest statement
Declaration of interests MS reports funding from National Institutes of Health during the conduct of this study. RB reports funds from Unitaid, United States Agency for International Development, and the Bill & Melinda Gates Foundation awarded to WHO. JDS reports receipt of HIV diagnostic device and test kits for conduct of Centers for Disease Control and Prevention-sponsored research from Diagnostics for the Real World. All other authors declare no conflicts of interests. The views expressed in this article do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Figures
Similar articles
-
Health impact, budget impact, and price threshold for cost-effectiveness of lenacapavir for HIV pre-exposure prophylaxis in eastern and southern Africa: a modelling analysis.Lancet HIV. 2024 Nov;11(11):e765-e773. doi: 10.1016/S2352-3018(24)00239-X. Epub 2024 Sep 20. Lancet HIV. 2024. PMID: 39312933 Free PMC article.
-
Uptake, engagement, and adherence to pre-exposure prophylaxis offered after population HIV testing in rural Kenya and Uganda: 72-week interim analysis of observational data from the SEARCH study.Lancet HIV. 2020 Apr;7(4):e249-e261. doi: 10.1016/S2352-3018(19)30433-3. Epub 2020 Feb 19. Lancet HIV. 2020. PMID: 32087152 Free PMC article.
-
Predicted effects of the introduction of long-acting injectable cabotegravir pre-exposure prophylaxis in sub-Saharan Africa: a modelling study.Lancet HIV. 2023 Apr;10(4):e254-e265. doi: 10.1016/S2352-3018(22)00365-4. Epub 2023 Jan 12. Lancet HIV. 2023. PMID: 36642087 Free PMC article.
-
The future of pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) infection.Expert Rev Anti Infect Ther. 2017 May;15(5):467-481. doi: 10.1080/14787210.2017.1309292. Epub 2017 Apr 4. Expert Rev Anti Infect Ther. 2017. PMID: 28322067 Review.
-
Kidney function in tenofovir disoproxil fumarate-based oral pre-exposure prophylaxis users: a systematic review and meta-analysis of published literature and a multi-country meta-analysis of individual participant data.Lancet HIV. 2022 Apr;9(4):e242-e253. doi: 10.1016/S2352-3018(22)00004-2. Epub 2022 Mar 7. Lancet HIV. 2022. PMID: 35271825 Free PMC article.
Cited by
-
Health and economic impact of oral PrEP provision across subgroups in western Kenya: a modelling analysis.BMJ Glob Health. 2025 Jan 11;10(1):e015835. doi: 10.1136/bmjgh-2024-015835. BMJ Glob Health. 2025. PMID: 39800385 Free PMC article.
-
HIV self-testing, PrEP, and drug resistance: some insights.Lancet HIV. 2024 Mar;11(3):e134-e136. doi: 10.1016/S2352-3018(23)00290-4. Epub 2024 Jan 29. Lancet HIV. 2024. PMID: 38301669 Free PMC article. No abstract available.
-
Preferences for pre-exposure prophylaxis delivery via online pharmacy among potential users in Kenya: a discrete choice experiment.J Int AIDS Soc. 2024 Oct;27(10):e26356. doi: 10.1002/jia2.26356. J Int AIDS Soc. 2024. PMID: 39385266 Free PMC article.
-
Estimated contributions and future mitigation strategies for HIV risk around funeral practices in western Kenya: a mathematical modeling study.BMC Med. 2025 Feb 12;23(1):85. doi: 10.1186/s12916-025-03907-4. BMC Med. 2025. PMID: 39939974 Free PMC article.
-
Breakthrough Acute HIV Infections among Pre-Exposure Prophylaxis Users with High Adherence: A Narrative Review.Viruses. 2024 Jun 12;16(6):951. doi: 10.3390/v16060951. Viruses. 2024. PMID: 38932243 Free PMC article. Review.
References
-
- Irungu EM, Baeten JM. PrEP rollout in Africa: status and opportunity. Nat Med. 2020;26:655–664. - PubMed
-
- WHO . World Health Organization; July 27, 2022. Differentiated and simplified pre-exposure prophylaxis for HIV prevention: update to WHO implementation guidance.https://www.who.int/publications/i/item/9789240053694
-
- WHO . World Health Organization; 2022. WHO public reports for in vitro diagnostics.https://extranet.who.int/pqweb/vitro-diagnostics/prequalification-report...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

