Repetitive head trauma and apoE4 induce chronic cerebrovascular alterations that impair tau elimination from the brain
- PMID: 38301863
- PMCID: PMC10922621
- DOI: 10.1016/j.expneurol.2024.114702
Repetitive head trauma and apoE4 induce chronic cerebrovascular alterations that impair tau elimination from the brain
Abstract
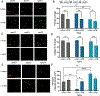
Repetitive mild traumatic brain injuries (r-mTBI) sustained in the military or contact sports have been associated with the accumulation of extracellular tau in the brain, which may contribute to the pathogenesis of neurodegenerative tauopathies. The expression of the apolipoprotein E4 (apoE4) isoform has been associated with higher levels of tau in the brain, and worse clinical outcomes after r-mTBI, though the influence of apoE genotype on extracellular tau dynamics in the brain is poorly understood. We recently demonstrated that extracellular tau can be eliminated across blood-brain barrier (BBB), which is progressively impaired following r-mTBI. The current studies investigated the influence of repetitive mild TBI (r-mTBI) and apoE genotype on the elimination of extracellular solutes from the brain. Following intracortical injection of biotin-labeled tau into humanized apoE-Tr mice, the levels of exogenous tau residing in the brain of apoE4 mice were elevated compared to other isoforms, indicating reduced tau elimination. Additionally, we found exposure to r-mTBI increased tau residence in apoE2 mice, similar to our observations in E2FAD animals. Each of these findings may be the result of diminished tau efflux via LRP1 at the BBB, as LRP1 inhibition significantly reduced tau uptake in endothelial cells and decreased tau transit across an in vitro model of the BBB (basolateral-to-apical). Notably, we showed that injury and apoE status, (particularly apoE4) resulted in chronic alterations in BBB integrity, pericyte coverage, and AQP4 polarization. These aberrations coincided with an atypical reactive astrocytic gene signature indicative of diminished CSF-ISF exchange. Our work found that CSF movement was reduced in the chronic phase following r-mTBI (>18 months post injury) across all apoE genotypes. In summary, we show that apoE genotype strongly influences cerebrovascular homeostasis, which can lead to age-dependent deficiencies in the elimination of toxic proteins from the brain, like tau, particularly in the aftermath of head trauma.
Keywords: ApoE; Blood brain barrier; Glymphatic system; Tau; Traumatic brain injury.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare they have no conflict of interest.
Figures
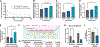



References
-
- Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Ech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D, 2018. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46 (W1), W537–W544. 10.1093/nar/gky379. - DOI - PMC - PubMed
-
- Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C, 2017. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 94 (3), 581–594.e5. 10.1016/j.neuron.2017.03.043. - DOI - PMC - PubMed
-
- Andrews S, 2010. FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. Available Online at. Http://Www.Bioinformatics.Babraham.Ac.Uk/Projects/Fastqc/.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

