Protein kinase C signaling "in" and "to" the nucleus: Master kinases in transcriptional regulation
- PMID: 38301892
- PMCID: PMC10907189
- DOI: 10.1016/j.jbc.2024.105692
Protein kinase C signaling "in" and "to" the nucleus: Master kinases in transcriptional regulation
Abstract
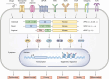
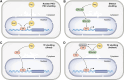
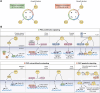
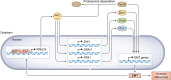
PKC is a multifunctional family of Ser-Thr kinases widely implicated in the regulation of fundamental cellular functions, including proliferation, polarity, motility, and differentiation. Notwithstanding their primary cytoplasmic localization and stringent activation by cell surface receptors, PKC isozymes impel prominent nuclear signaling ultimately impacting gene expression. While transcriptional regulation may be wielded by nuclear PKCs, it most often relies on cytoplasmic phosphorylation events that result in nuclear shuttling of PKC downstream effectors, including transcription factors. As expected from the unique coupling of PKC isozymes to signaling effector pathways, glaring disparities in gene activation/repression are observed upon targeting individual PKC family members. Notably, specific PKCs control the expression and activation of transcription factors implicated in cell cycle/mitogenesis, epithelial-to-mesenchymal transition and immune function. Additionally, PKCs isozymes tightly regulate transcription factors involved in stepwise differentiation of pluripotent stem cells toward specific epithelial, mesenchymal, and hematopoietic cell lineages. Aberrant PKC expression and/or activation in pathological conditions, such as in cancer, leads to profound alterations in gene expression, leading to an extensive rewiring of transcriptional networks associated with mitogenesis, invasiveness, stemness, and tumor microenvironment dysregulation. In this review, we outline the current understanding of PKC signaling "in" and "to" the nucleus, with significant focus on established paradigms of PKC-mediated transcriptional control. Dissecting these complexities would allow the identification of relevant molecular targets implicated in a wide spectrum of diseases.
Keywords: diacylglycerol; gene expression; nucleus; protein kinase C; signal transduction; transcription factor.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- Newton A.C. Regulation of protein kinase C. Curr. Opin. Cell Biol. 1997;9:161–167. - PubMed
-
- Toker A. Signaling through protein kinase C. Front. Biosci. 1998;3:D1134–D1147. - PubMed
-
- Griner E.M., Kazanietz M.G. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer. 2007;7:281–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

