Discovery and Characterization of VU0542270, the First Selective Inhibitor of Vascular Kir6.1/SUR2B KATP Channels
- PMID: 38302135
- PMCID: PMC10877733
- DOI: 10.1124/molpharm.123.000783
Discovery and Characterization of VU0542270, the First Selective Inhibitor of Vascular Kir6.1/SUR2B KATP Channels
Abstract
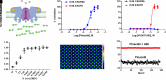
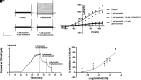
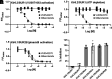
Vascular smooth muscle KATP channels critically regulate blood flow and blood pressure by modulating vascular tone and therefore represent attractive drug targets for treating several cardiovascular disorders. However, the lack of potent inhibitors that can selectively inhibit Kir6.1/SUR2B (vascular KATP) over Kir6.2/SUR1 (pancreatic KATP) has eluded discovery despite decades of intensive research. We therefore screened 47,872 chemically diverse compounds for novel inhibitors of heterologously expressed Kir6.1/SUR2B channels. The most potent inhibitor identified in the screen was an N-aryl-N'-benzyl urea compound termed VU0542270. VU0542270 inhibits Kir6.1/SUR2B with an IC50 of approximately 100 nM but has no apparent activity toward Kir6.2/SUR1 or several other members of the Kir channel family at doses up to 30 µM (>300-fold selectivity). By expressing different combinations of Kir6.1 or Kir6.2 with SUR1, SUR2A, or SUR2B, the VU0542270 binding site was localized to SUR2. Initial structure-activity relationship exploration around VU0542270 revealed basic texture related to structural elements that are required for Kir6.1/SUR2B inhibition. Analysis of the pharmacokinetic properties of VU0542270 showed that it has a short in vivo half-life due to extensive metabolism. In pressure myography experiments on isolated mouse ductus arteriosus vessels, VU0542270 induced ductus arteriosus constriction in a dose-dependent manner similar to that of the nonspecific KATP channel inhibitor glibenclamide. The discovery of VU0542270 provides conceptual proof that SUR2-specific KATP channel inhibitors can be developed using a molecular target-based approach and offers hope for developing cardiovascular therapeutics targeting Kir6.1/SUR2B. SIGNIFICANCE STATEMENT: Small-molecule inhibitors of vascular smooth muscle KATP channels might represent novel therapeutics for patent ductus arteriosus, migraine headache, and sepsis; however, the lack of selective channel inhibitors has slowed progress in these therapeutic areas. Here, this study describes the discovery and characterization of the first vascular-specific KATP channel inhibitor, VU0542270.
Copyright © 2024 by The Author(s).
Figures

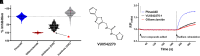

Similar articles
-
Characterization of four structurally diverse inhibitors of SUR2-containing KATP channels.Channels (Austin). 2024 Dec;18(1):2398565. doi: 10.1080/19336950.2024.2398565. Epub 2024 Sep 20. Channels (Austin). 2024. PMID: 39303216 Free PMC article.
-
Effects of ZD0947, a novel and potent ATP-sensitive K+ channel opener, on smooth muscle-type ATP-sensitive K+ channels.Eur J Pharmacol. 2016 Nov 15;791:773-779. doi: 10.1016/j.ejphar.2016.09.038. Epub 2016 Sep 29. Eur J Pharmacol. 2016. PMID: 27693800
-
Kir6.1-dependent KATP channels in lymphatic smooth muscle and vessel dysfunction in mice with Kir6.1 gain-of-function.J Physiol. 2020 Aug;598(15):3107-3127. doi: 10.1113/JP279612. Epub 2020 May 30. J Physiol. 2020. PMID: 32372450 Free PMC article.
-
ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells.Am J Physiol. 1998 Jan;274(1):C25-37. doi: 10.1152/ajpcell.1998.274.1.C25. Am J Physiol. 1998. PMID: 9458709 Review.
-
K(ATP) channel action in vascular tone regulation: from genetics to diseases.Sheng Li Xue Bao. 2012 Feb 25;64(1):1-13. Sheng Li Xue Bao. 2012. PMID: 22348955 Free PMC article. Review.
Cited by
-
Automated patch clamp analysis of heterologously expressed Kir6.2/SUR1 and Kir6.1/SUR2B KATP currents.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C82-C92. doi: 10.1152/ajpcell.00266.2025. Epub 2025 Jun 4. Am J Physiol Cell Physiol. 2025. PMID: 40465479 Free PMC article.
-
Modulatory Impact of Oxidative Stress on Action Potentials in Pathophysiological States: A Comprehensive Review.Antioxidants (Basel). 2024 Sep 26;13(10):1172. doi: 10.3390/antiox13101172. Antioxidants (Basel). 2024. PMID: 39456426 Free PMC article. Review.
-
Patent Ductus Arteriosus in Extremely Preterm Infants: Update on Current Diagnostic and Treatment Options.Curr Treat Options Cardiovasc Med. 2025;27(1):43. doi: 10.1007/s11936-025-01101-6. Epub 2025 Jul 22. Curr Treat Options Cardiovasc Med. 2025. PMID: 40709290 Free PMC article. Review.
-
Characterization of four structurally diverse inhibitors of SUR2-containing KATP channels.Channels (Austin). 2024 Dec;18(1):2398565. doi: 10.1080/19336950.2024.2398565. Epub 2024 Sep 20. Channels (Austin). 2024. PMID: 39303216 Free PMC article.
References
-
- Aguilar-Bryan L, Bryan J (1999) Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20:101–135. - PubMed
-
- Ashfield R, Gribble FM, Ashcroft SJ, Ashcroft FM (1999) Identification of the high-affinity tolbutamide site on the SUR1 subunit of the K(ATP) channel. Diabetes 48:1341–1347. - PubMed
-
- Aziz Q, Thomas AM, Gomes J, Ang R, Sones WR, Li Y, Ng KE, Gee L, Tinker A (2014) The ATP-sensitive potassium channel subunit, Kir6.1, in vascular smooth muscle plays a major role in blood pressure control. Hypertension 64:523–529. - PubMed
-
- Brownstein CATowne MCLuquette LJHarris DJMarinakis NSMeinecke PKutsche KCampeau PMYu TWMargulies DM, et al. (2013) Mutation of KCNJ8 in a patient with Cantú syndrome with unique vascular abnormalities - support for the role of K(ATP) channels in this condition. Eur J Med Genet 56:678–682. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

