Cystic fibrosis in Europe: improved lung function and longevity - reasons for cautious optimism, but challenges remain
- PMID: 38302155
- PMCID: PMC10918317
- DOI: 10.1183/13993003.01241-2023
Cystic fibrosis in Europe: improved lung function and longevity - reasons for cautious optimism, but challenges remain
Abstract
Background: Prognosis and disease severity in cystic fibrosis (CF) are linked to declining lung function. To characterise lung function by the number of adults in countries with different levels of Gross National Income (GNI), data from the European Cystic Fibrosis Society Patient Registry were utilised.
Methods: Annual data including age, forced expiratory volume in 1 s (FEV1), anthropometry, genotype, respiratory cultures and CF-related diabetes (CFRD) were retrieved between 2011 and 2021. All countries were stratified into GNI per capita to reflect differences within Europe.
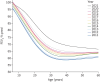
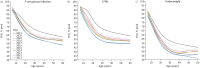
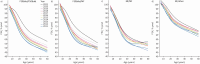
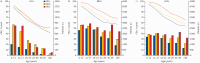
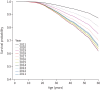
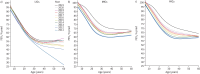
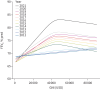
Results: A consistent improvement in FEV1 % pred and survival was observed among the 47 621 people with CF (pwCF), including subjects with chronic Pseudomonas aeruginosa infection, CFRD and/or undernutrition. Mean values of FEV1 % pred changed from 85% to 94.2% for children and from 63.6% to 74.7% for adults. FEV1 % pred further increased among those carrying the F508del mutation in 2021, when elexacaftor/tezacaftor/ivacaftor was available. The number of adult pwCF increased from 13 312 in 2011 to 21 168 in 2021, showing a 60% increase. PwCF living in European lower income countries did not demonstrate a significant annual increase in FEV1 % pred or in the number of adults.
Conclusion: This pan-European analysis demonstrates a consistent improvement in FEV1 % pred, number of adult pwCF and survival over the last decade only in European higher and middle income countries. Urgent action is needed in the lower income countries where such improvement was not observed. The notable improvement observed in pwCF carrying the F508del mutation emphasises the need to develop treatments for all CF mutations.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: L. Naehrlich reports grants from the German Center for Lung Research, Vertex Pharmaceuticals and Mukoviszidose Institute, participation on a trial steering committee for CF STORM, leadership roles as medical lead of the German CF Registry and pharmacovigilance study manager of the ECFSPR, and medical writing from Articulate Science, outside the submitted work. I. Sermet-Gaudelus reports grants, consulting fees and lecture honoraria from Vertex Pharmaceuticals, and a leadership role as medical lead of the French Pediatric CF Network, outside the submitted work. The remaining authors have no potential conflicts of interest to disclose.
Figures
Comment in
-
Socioeconomic disparities in European cystic fibrosis outcomes: time to close the gap.Eur Respir J. 2024 Mar 7;63(3):2400328. doi: 10.1183/13993003.00328-2024. Print 2024 Mar. Eur Respir J. 2024. PMID: 38453247 No abstract available.
Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
-
Nonsense mutations accelerate lung disease and decrease survival of cystic fibrosis children.J Cyst Fibros. 2023 Nov;22(6):1070-1079. doi: 10.1016/j.jcf.2023.06.005. Epub 2023 Jul 6. J Cyst Fibros. 2023. PMID: 37422433
-
Who are the 10%? - Non eligibility of cystic fibrosis (CF) patients for highly effective modulator therapies.Respir Med. 2022 Aug;199:106878. doi: 10.1016/j.rmed.2022.106878. Epub 2022 May 16. Respir Med. 2022. PMID: 35633605
-
Real-world data on the efficacy and safety of tezacaftor-ivacaftor in adults living with cystic fibrosis homozygous for F508del and heterozygous for F508del and a residual function mutation.Acta Clin Belg. 2023 Aug;78(4):280-284. doi: 10.1080/17843286.2022.2145684. Epub 2022 Nov 22. Acta Clin Belg. 2023. PMID: 36415912
-
Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2019 Jan 7;1(1):CD009841. doi: 10.1002/14651858.CD009841.pub3. Cochrane Database Syst Rev. 2019. PMID: 30616300 Free PMC article.
Cited by
-
Expanding the Impact of New Cystic Fibrosis Therapies in Low- and Middle-Income Countries.Pediatr Pulmonol. 2025 Mar;60 Suppl 1(Suppl 1):S90-S91. doi: 10.1002/ppul.27362. Epub 2024 Dec 3. Pediatr Pulmonol. 2025. PMID: 39625248 Free PMC article. Review.
-
Overcoming Pseudomonas aeruginosa in Chronic Suppurative Lung Disease: Prevalence, Treatment Challenges, and the Promise of Bacteriophage Therapy.Antibiotics (Basel). 2025 Apr 23;14(5):427. doi: 10.3390/antibiotics14050427. Antibiotics (Basel). 2025. PMID: 40426494 Free PMC article. Review.
-
Cystic Fibrosis Transmembrane Conductance Regulator Modulators: Real-World Evidence Highlights Need for Worldwide Access.Ann Am Thorac Soc. 2024 Jul;21(7):1003-1004. doi: 10.1513/AnnalsATS.202404-439ED. Ann Am Thorac Soc. 2024. PMID: 38949605 Free PMC article. No abstract available.
-
Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Cystic Fibrosis: A Review of Registry-Based Evidence.J Clin Med. 2025 Jun 5;14(11):3978. doi: 10.3390/jcm14113978. J Clin Med. 2025. PMID: 40507740 Free PMC article. Review.
-
Respiratory infections after elexacaftor/tezacaftor/ivacaftor treatment in people with cystic fibrosis: analysis of the European Cystic Fibrosis Society Patient Registry.ERJ Open Res. 2025 Aug 4;11(4):01248-2024. doi: 10.1183/23120541.01248-2024. eCollection 2025 Jul. ERJ Open Res. 2025. PMID: 40761658 Free PMC article.
