Neuronal DSCAM regulates the peri-synaptic localization of GLAST in Bergmann glia for functional synapse formation
- PMID: 38302444
- PMCID: PMC10834496
- DOI: 10.1038/s41467-023-44579-z
Neuronal DSCAM regulates the peri-synaptic localization of GLAST in Bergmann glia for functional synapse formation
Abstract
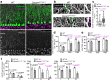
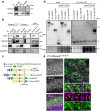
In the central nervous system, astrocytes enable appropriate synapse function through glutamate clearance from the synaptic cleft; however, it remains unclear how astrocytic glutamate transporters function at peri-synaptic contact. Here, we report that Down syndrome cell adhesion molecule (DSCAM) in Purkinje cells controls synapse formation and function in the developing cerebellum. Dscam-mutant mice show defects in CF synapse translocation as is observed in loss of function mutations in the astrocytic glutamate transporter GLAST expressed in Bergmann glia. These mice show impaired glutamate clearance and the delocalization of GLAST away from the cleft of parallel fibre (PF) synapse. GLAST complexes with the extracellular domain of DSCAM. Riluzole, as an activator of GLAST-mediated uptake, rescues the proximal impairment in CF synapse formation in Purkinje cell-selective Dscam-deficient mice. DSCAM is required for motor learning, but not gross motor coordination. In conclusion, the intercellular association of synaptic and astrocyte proteins is important for synapse formation and function in neural transmission.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
- JP22wm0425005/Japan Agency for Medical Research and Development (AMED)
- JP23wm0425005h0003/Japan Agency for Medical Research and Development (AMED)
- JP23wm0425005h0003/Japan Agency for Medical Research and Development (AMED)
- #JP17J07118/MEXT | Japan Society for the Promotion of Science (JSPS)
- JPMJCR21P1; JPMJMS2292/MEXT | JST | Core Research for Evolutional Science and Technology (CREST)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

