What is the pipeline for future medications for obesity?
- PMID: 38302593
- PMCID: PMC11971045
- DOI: 10.1038/s41366-024-01473-y
What is the pipeline for future medications for obesity?
Abstract
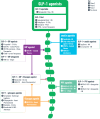
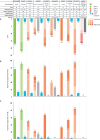
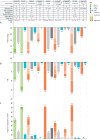
Obesity is a chronic disease associated with increased risk of obesity-related complications and mortality. Our better understanding of the weight regulation mechanisms and the role of gut-brain axis on appetite has led to the development of safe and effective entero-pancreatic hormone-based treatments for obesity such as glucagon-like peptide-1 (GLP-1) receptor agonists (RA). Semaglutide 2.4 mg once weekly, a subcutaneously administered GLP-1 RA approved for obesity treatment in 2021, results in 15-17% mean weight loss (WL) with evidence of cardioprotection. Oral GLP-1 RA are also under development and early data shows similar WL efficacy to semaglutide 2.4 mg. Looking to the next generation of obesity treatments, combinations of GLP-1 with other entero-pancreatic hormones with complementary actions and/or synergistic potential (such as glucose-dependent insulinotropic polypeptide (GIP), glucagon, and amylin) are under investigation to enhance the WL and cardiometabolic benefits of GLP-1 RA. Tirzepatide, a dual GLP-1/GIP receptor agonist has been approved for glycaemic control in type 2 diabetes as well as for obesity management leading in up to 22.5% WL in phase 3 obesity trials. Other combinations of entero-pancreatic hormones including cagrisema (GLP-1/amylin RA) and the triple agonist retatrutide (GLP-1/GIP/glucagon RA) have also progressed to phase 3 trials as obesity treatments and early data suggests that may lead to even greater WL than tirzepatide. Additionally, agents with different mechanisms of action to entero-pancreatic hormones (e.g. bimagrumab) may improve the body composition during WL and are in early phase clinical trials. We are in a new era for obesity pharmacotherapy where combinations of entero-pancreatic hormones approach the WL achieved with bariatric surgery. In this review, we present the efficacy and safety data for the pipeline of obesity pharmacotherapies with a focus on entero-pancreatic hormone-based treatments and we consider the clinical implications and challenges that the new era in obesity management may bring.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: E.M. and U.A. declare no competing interest. D.P. has acted as a speaker for Novo Nordisk and has received grants from Novo Nordisk, Novo Nordisk UK Research Foundation, Academy of Medical Sciences/ Diabetes UK and Health Education East Midlands. M.J.D. has acted as consultant, advisory board member and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi, an advisory board member Lexicon, Pfizer, ShouTi Pharma Inc, AstraZeneca and Medtronic and as a speaker for AstraZeneca, Napp Pharmaceuticals, Novartis and Amgen. M.J.D has received grants from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Janssen and Sanofi-Aventis and Eli Lilly.
Figures

References
-
- Busetto L, Bettini S, Makaronidis J, Roberts CA, Halford JCG, Batterham RL. Mechanisms of weight regain. Eur J Intern Med. 2021;93:3–7. - PubMed
-
- Kinlen D, Cody D, O’Shea D. Complications of obesity. QJM. 2018;111:437–43. - PubMed
-
- Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

