Drug-drug interaction assessment based on a large-scale spontaneous reporting system for hepato- and renal-toxicity, and thrombocytopenia with concomitant low-dose methotrexate and analgesics use
- PMID: 38303016
- PMCID: PMC10832291
- DOI: 10.1186/s40360-024-00738-6
Drug-drug interaction assessment based on a large-scale spontaneous reporting system for hepato- and renal-toxicity, and thrombocytopenia with concomitant low-dose methotrexate and analgesics use
Abstract
Background: Methotrexate (MTX) is the cornerstone of rheumatoid arthritis (RA) treatment and is highly effective with low-dose intermittent administration. MTX is occasionally used in combination with non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen (APAP)/paracetamol for pain or inflammation control. With MTX treatment, the side effects, such as hepatotoxicity, renal failure, and myelosuppression should be considered. These are also seen with analgesics treatment.
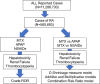
Methods: We used a large spontaneously reported adverse event database (FAERS [JAPIC AERS]) to analyze whether the reporting of adverse events increased upon MTX and analgesic therapy in patients with RA.
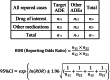
Results: After identifying RA cases, the crude reporting odds ratios (cRORs) for hepatotoxicity, renal failure, and thrombocytopenia associated with the use of MTX, APAP, or NSAIDs were calculated by disproportionality analysis, which revealed significantly higher cRORs for these events. No analgesics showed consistent positive signals for drug-drug interaction (DDI) with concomitant low-dose MTX analyzed using four algorithms for DDI interaction (the Ω shrinkage measure, additive or multiplicative, and combination risk ratio models). However, in renal failure and thrombocytopenia, loxoprofen (Ω025 = 0.08) and piroxicam (Ω025 = 0.46), and ibuprofen (Ω025 = 0.74) and ketorolac (Ω025 = 3.52), respectively, showed positive signals in the Ω shrinkage measure model, and no consistency was found among adverse events or NSAIDs.
Conclusions: Studies using spontaneous reporting systems have limitations such as reporting bias or lack of patient background; however, the results of our comprehensive analysis support the results of previous clinical or epidemiological studies. This study also demonstrated the usefulness of FAERS for DDI assessment.
Keywords: Acetaminophen; Drug-drug interaction; FAERS; Hepatotoxicity; Methotrexate; NSAIDs; Renaltoxicity; Rheumatoid arthritis; Thrombocytopenia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

