The SATB1-MIR22-GBA axis mediates glucocerebroside accumulation inducing a cellular senescence-like phenotype in dopaminergic neurons
- PMID: 38303548
- PMCID: PMC11019121
- DOI: 10.1111/acel.14077
The SATB1-MIR22-GBA axis mediates glucocerebroside accumulation inducing a cellular senescence-like phenotype in dopaminergic neurons
Abstract
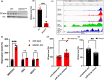
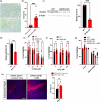
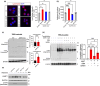
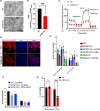
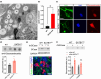
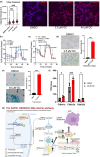
Idiopathic Parkinson's disease (PD) is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta, which is associated with neuroinflammation and reactive gliosis. The underlying cause of PD and the concurrent neuroinflammation are not well understood. In this study, we utilize human and murine neuronal lines, stem cell-derived dopaminergic neurons, and mice to demonstrate that three previously identified genetic risk factors for PD, namely SATB1, MIR22HG, and GBA, are components of a single gene regulatory pathway. Our findings indicate that dysregulation of this pathway leads to the upregulation of glucocerebrosides (GluCer), which triggers a cellular senescence-like phenotype in dopaminergic neurons. Specifically, we discovered that downregulation of the transcriptional repressor SATB1 results in the derepression of the microRNA miR-22-3p, leading to decreased GBA expression and subsequent accumulation of GluCer. Furthermore, our results demonstrate that an increase in GluCer alone is sufficient to impair lysosomal and mitochondrial function, thereby inducing cellular senescence. Dysregulation of the SATB1-MIR22-GBA pathway, observed in both PD patients and normal aging, leads to lysosomal and mitochondrial dysfunction due to the GluCer accumulation, ultimately resulting in a cellular senescence-like phenotype in dopaminergic neurons. Therefore, our study highlights a novel pathway involving three genetic risk factors for PD and provides a potential mechanism for the senescence-induced neuroinflammation and reactive gliosis observed in both PD and normal aging.
Keywords: MicroRNAs; Parkinson's disease; gene expression regulation; glucosylceramides; lysosomes; mitochondria; neuroinflammation; senescence.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
L.S. is a scientific cofounder and paid consultant of BlueRock Therapeutics Inc, and a scientific cofounder of DaCapo Brainscience.
Figures
Update of
-
The SATB1-MIR22-GBA axis mediates glucocerebroside accumulation inducing a cellular senescence-like phenotype in dopaminergic neurons.bioRxiv [Preprint]. 2023 Jul 21:2023.07.19.549710. doi: 10.1101/2023.07.19.549710. bioRxiv. 2023. Update in: Aging Cell. 2024 Apr;23(4):e14077. doi: 10.1111/acel.14077. PMID: 37503189 Free PMC article. Updated. Preprint.
References
-
- Beutler, E. (1992). Gaucher disease: New molecular approaches to diagnosis and treatment. Science, 256(5058), 794–799. - PubMed
-
- Brichta, L. , Shin, W. , Jackson‐Lewis, V. , Blesa, J. , Yap, E. L. , Walker, Z. , Zhang, J. , Roussarie, J. P. , Alvarez, M. J. , Califano, A. , Przedborski, S. , & Greengard, P. (2015). Identification of neurodegenerative factors using translatome‐regulatory network analysis. Nature Neuroscience, 18(9), 1325–1333. 10.1038/nn.4070 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

