High resolution and contrast 7 tesla MR brain imaging of the neonate
- PMID: 38304343
- PMCID: PMC10830693
- DOI: 10.3389/fradi.2023.1327075
High resolution and contrast 7 tesla MR brain imaging of the neonate
Abstract
Introduction: Ultra-high field MR imaging offers marked gains in signal-to-noise ratio, spatial resolution, and contrast which translate to improved pathological and anatomical sensitivity. These benefits are particularly relevant for the neonatal brain which is rapidly developing and sensitive to injury. However, experience of imaging neonates at 7T has been limited due to regulatory, safety, and practical considerations. We aimed to establish a program for safely acquiring high resolution and contrast brain images from neonates on a 7T system.
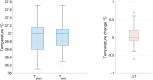
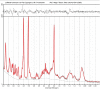
Methods: Images were acquired from 35 neonates on 44 occasions (median age 39 + 6 postmenstrual weeks, range 33 + 4 to 52 + 6; median body weight 2.93 kg, range 1.57 to 5.3 kg) over a median time of 49 mins 30 s. Peripheral body temperature and physiological measures were recorded throughout scanning. Acquired sequences included T2 weighted (TSE), Actual Flip angle Imaging (AFI), functional MRI (BOLD EPI), susceptibility weighted imaging (SWI), and MR spectroscopy (STEAM).
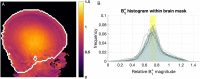
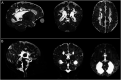
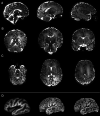
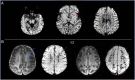
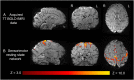
Results: There was no significant difference between temperature before and after scanning (p = 0.76) and image quality assessment compared favorably to state-of-the-art 3T acquisitions. Anatomical imaging demonstrated excellent sensitivity to structures which are typically hard to visualize at lower field strengths including the hippocampus, cerebellum, and vasculature. Images were also acquired with contrast mechanisms which are enhanced at ultra-high field including susceptibility weighted imaging, functional MRI, and MR spectroscopy.
Discussion: We demonstrate safety and feasibility of imaging vulnerable neonates at ultra-high field and highlight the untapped potential for providing important new insights into brain development and pathological processes during this critical phase of early life.
Keywords: brain; infant; magnetic resonance imaging (MRI); neonate; neuroradiology; ultra-high field MRI.
© 2024 Bridgen, Tomi-Tricot, Uus, Cromb, Quirke, Almalbis, Bonse, De la Fuente Botella, Maggioni, Cio, Cawley, Casella, Dokumaci, Thomson, Willers Moore, Bridglal, Saravia, Finck, Price, Pickles, Cordero-Grande, Egloff, O'Muircheartaigh, Counsell, Giles, Deprez, De Vita, Rutherford, Edwards, Hajnal, Malik and Arichi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

