Optimization of Growth Conditions for Chlorpyrifos-Degrading Bacteria in Farm Soils in Nakuru County, Kenya
- PMID: 38304346
- PMCID: PMC10834098
- DOI: 10.1155/2024/1611871
Optimization of Growth Conditions for Chlorpyrifos-Degrading Bacteria in Farm Soils in Nakuru County, Kenya
Abstract
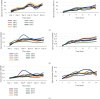
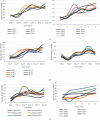
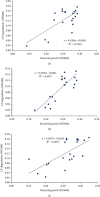
Chlorpyrifos (CP) is a chlorinated organophosphate pesticide. In Kenya, it is commonly used as an acaricide, particularly in dairy farming, leading to soil and water contamination. The study is aimed at isolating bacteria with CP-degrading potential and optimizing their growth conditions, including temperature, pH, and CP concentration. The enrichment culture technique was used, with minimal salt medium (MSM) supplemented with commercial grade CP. A multilevel factorial design was used to investigate the interactions of temperature, pH, and CP concentration. According to the findings, seven bacterial strains with potential to degrade CP were characterized and identified as Alcaligenes faecalis, Bacillus weihenstephanensis, Bacillus toyonensis, Alcaligenes sp. strain SCAU23, Pseudomonas sp. strain PB845W, Brevundimonas diminuta, and uncultured bacterium clone 99. Growth and biodegradation of bacteria differed significantly among the isolates across pH value, temperature, and concentrations (P ≤ 0.05). The optimum conditions for growth were pH 7, temperature of 25°C, and 25mg/l chlorpyrifos concentration, while optimum degradation conditions were pH 5, temp 25°C, and CP conc. 25mg/l. The Pearson correlation between optimum growth and degradation showed a weak positive relationship (R = 0.1144) for pH and strong positive relationship for temperature and concentration of chlorpyrifos. Other than pH, the study shows that there could be other cofactors facilitating the chlorpyrifos degradation process. The findings show that an efficient consortium, at 25°C and pH 5, can include Bacillus toyonensis 20SBZ2B and Alcaligenes sp. SCAU23 as they showed high optical density (OD) values under these conditions. These results indicate the potential for these bacteria to be employed in chlorpyrifos-contaminated ecosystem detoxification efforts upon manipulation of natural growth conditions. The findings of this study offer a potential foundation for future research into the reconstitution of a consortium. Based on the optimum conditions identified, the isolated bacterial strains could be further developed into a consortium to effectively degrade CP in both laboratory and field conditions. Dairy farmers can utilize the isolated strains and the consortia to decontaminate farm soils.
Copyright © 2024 Miriam Wepukhulu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Molecular characterization of chlorpyrifos degrading bacteria isolated from contaminated dairy farm soils in Nakuru County, Kenya.Heliyon. 2022 Mar 24;8(3):e09176. doi: 10.1016/j.heliyon.2022.e09176. eCollection 2022 Mar. Heliyon. 2022. PMID: 35846483 Free PMC article.
-
New insights into the biodegradation of chlorpyrifos by a novel bacterial consortium: Process optimization using general factorial experimental design.Ecotoxicol Environ Saf. 2021 Feb;209:111799. doi: 10.1016/j.ecoenv.2020.111799. Epub 2020 Dec 24. Ecotoxicol Environ Saf. 2021. PMID: 33360782
-
Bacterial community analysis in chlorpyrifos enrichment cultures via DGGE and use of bacterial consortium for CP biodegradation.World J Microbiol Biotechnol. 2014 Oct;30(10):2755-66. doi: 10.1007/s11274-014-1699-8. Epub 2014 Jul 10. World J Microbiol Biotechnol. 2014. PMID: 25008559
-
Utilization of microbial community potential for removal of chlorpyrifos: a review.Crit Rev Biotechnol. 2016 Aug;36(4):727-42. doi: 10.3109/07388551.2015.1015958. Epub 2015 Mar 18. Crit Rev Biotechnol. 2016. PMID: 25782532 Review.
-
Environmental Distribution, Metabolic Fate, and Degradation Mechanism of Chlorpyrifos: Recent and Future Perspectives.Appl Biochem Biotechnol. 2022 May;194(5):2301-2335. doi: 10.1007/s12010-021-03713-7. Epub 2022 Jan 11. Appl Biochem Biotechnol. 2022. PMID: 35013924 Review.
Cited by
-
Engineering Escherichia coli via introduction of the isopentenol utilization pathway to effectively produce geranyllinalool.Microb Cell Fact. 2024 Oct 24;23(1):292. doi: 10.1186/s12934-024-02563-2. Microb Cell Fact. 2024. PMID: 39443997 Free PMC article.
References
-
- Sharma P., Gaur N. Microbial biopesticides use in insect-pest management: an overview. Microbial Biotechnology in Crop Protection . 2021:123–145. doi: 10.1007/978-981-16-0049-4_5. - DOI
-
- Abong’o D. A., Wandiga S. O., Jumba I. O., Madadi V. O., Kylin H. Impacts of pesticides on human health and environment in the river Nyando catchment, Kenya . Erepository.uonbi.ac.ke; 2014. http://erepository.uonbi.ac.ke/handle/11295/72981 .
-
- Poudel S., Poudel B., Acharya B., Poudel P. Pesticide use and its impacts on human health and environment. Environment & Ecosystem Science . 2020;4(1):47–51. doi: 10.26480/ees.01.2020.47.51. - DOI
-
- Bhende R. S., Jhariya U., Srivastava S., Bombaywala S., Das S., Dafale N. A. Environmental distribution, metabolic fate, and degradation mechanism of chlorpyrifos: recent and future perspectives. Applied Biochemistry and Biotechnology . 2022;194(5):2301–2335. doi: 10.1007/s12010-021-03713-7. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

