Immunogenicity studies of recombinant RBD SARS-CoV-2 as a COVID-19 vaccine candidate produced in Escherichia coli
- PMID: 38304876
- PMCID: PMC10832452
- DOI: 10.1016/j.jvacx.2024.100443
Immunogenicity studies of recombinant RBD SARS-CoV-2 as a COVID-19 vaccine candidate produced in Escherichia coli
Abstract
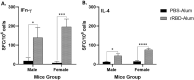
The severe acute respiratory syndrome coronavirus 2 -related global COVID-19 pandemic has been impacting millions of people since its outbreak in 2020. COVID-19 vaccination has proven highly efficient in reducing illness severity and preventing infection-related fatalities. The World Health Organization has granted emergency use approval to multiple, including protein subunit technology-based, COVID-19 vaccines. Foreseeably, additional COVID-19 subunit vaccine development would be essential to meet the accessible and growing demand for effective vaccines, especially for Low-Middle-Income Countries (LMIC). The SARS-CoV-2 spike protein receptor binding domain (RBD), as the primary target for neutralizing antibodies, holds significant potential for future COVID-19 subunit vaccine development. In this study, we developed a recombinant Escherichia coli-expressed RBD (rRBD) as a vaccine candidate and evaluated its immunogenicity and preliminary toxicity in BALB/c mice. The rRBD induced humoral immune response from day 7 post-vaccination and, following the booster doses, the IgG levels increased dramatically in mice. Interestingly, our vaccine candidate also significantly induced cellular immune response, indicated by the incrased IFN-ɣ-producing cell numbers. We observed no adverse effect or local reactogenicity either in control or treated mice. Taken together, our discoveries could potentially support efficient and cost-effective vaccine antigen production, from which LMICs could particularly benefit.
Keywords: COVID-19; Escherichia coli; RBD; SARS-CoV-2; Vaccine candidate.
© 2024 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dessy Natalia reports equipment, drugs, or supplies was provided by Indonesia Endowment Fund for Education. Marselina Irasonia Tan reports equipment, drugs, or supplies was provided by Indonesia Endowment Fund for Education. Dessy Natalia reports equipment, drugs, or supplies was provided by National Research and Innovation Agency Republic of Indonesia. Marselina Irasonia Tan reports equipment, drugs, or supplies was provided by National Research and Innovation Agency Republic of Indonesia.
Figures



References
-
- Piccoli L, Park YJ, Tortorici MA, Czudnochowski N, Walls AC, Beltramello M, et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell. 2020;183 doi: 10.1016/j.cell.2020.09.037. 1024–1042.e1021. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

