Identification of druggable host dependency factors shared by multiple SARS-CoV-2 variants of concern
- PMID: 38305139
- PMCID: PMC11411213
- DOI: 10.1093/jmcb/mjae004
Identification of druggable host dependency factors shared by multiple SARS-CoV-2 variants of concern
Abstract
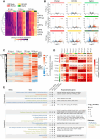
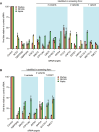
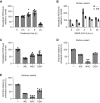
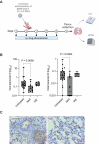
The high mutation rate of SARS-CoV-2 leads to the emergence of multiple variants, some of which are resistant to vaccines and drugs targeting viral elements. Targeting host dependency factors, e.g. cellular proteins required for viral replication, would help prevent the development of resistance. However, it remains unclear whether different SARS-CoV-2 variants induce conserved cellular responses and exploit the same core host factors. To this end, we compared three variants of concern and found that the host transcriptional response was conserved, differing only in kinetics and magnitude. Clustered regularly interspaced short palindromic repeats screening identified host genes required for each variant during infection. Most of the genes were shared by multiple variants. We validated our hits with small molecules and repurposed the US Food and Drug Administration-approved drugs. All the drugs were highly active against all the tested variants, including new variants that emerged during the study (Delta and Omicron). Mechanistically, we identified reactive oxygen species production as a key step in early viral replication. Antioxidants such as N-acetyl cysteine (NAC) were effective against all the variants in both human lung cells and a humanized mouse model. Our study supports the use of available antioxidant drugs, such as NAC, as a general and effective anti-COVID-19 approach.
Keywords: N-acetyl cysteine; SARS-CoV-2; antivirals; host dependency factors; variants of concern.
© The Author(s) (2024). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures


References
-
- Baggen J., Persoons L., Vanstreels E. et al. (2021). Genome-wide CRISPR screening identifies TMEM106B as a proviral host factor for SARS-CoV-2. Nat. Genet. 53, 435–444. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

