The 25-kDa linear polyethylenimine exerts specific antiviral activity against pseudorabies virus through interferencing its adsorption via electrostatic interaction
- PMID: 38305153
- PMCID: PMC10949462
- DOI: 10.1128/jvi.00007-24
The 25-kDa linear polyethylenimine exerts specific antiviral activity against pseudorabies virus through interferencing its adsorption via electrostatic interaction
Abstract
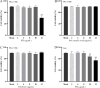
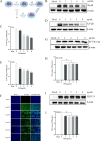
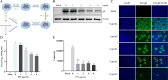
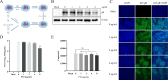
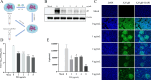
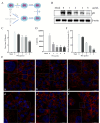
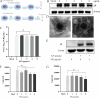
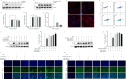
Pseudorabies virus (PRV) is the causative agent of Aujeszky's disease, which is responsible for enormous economic losses to the global pig industry. Although vaccination has been used to prevent PRV infection, the effectiveness of vaccines has been greatly diminished with the emergence of PRV variants. Therefore, there is an urgent need to develop anti-PRV drugs. Polyethylenimine (PEI) is a cationic polymer and has a wide range of antibacterial and antiviral activities. This study found that a low dose of 1 µg/mL of the 25-kDa linear PEI had significantly specific anti-PRV activity, which became more intense with increasing concentrations. Mechanistic studies revealed that the viral adsorption stage was the major target of PEI without affecting viral entry, replication stages, and direct inactivation effects. Subsequently, we found that cationic polymers PEI and Polybrene interfered with the interaction between viral proteins and cell surface receptors through electrostatic interaction to exert the antiviral function. In conclusion, cationic polymers such as PEI can be a category of options for defense against PRV. Understanding the anti-PRV mechanism also deepens host-virus interactions and reveals new drug targets for anti-PRV.IMPORTANCEPolyethylenimine (PEI) is a cationic polymer that plays an essential role in the host immune response against microbial infections. However, the specific mechanisms of PEI in interfering with pseudorabies virus (PRV) infection remain unclear. Here, we found that 25-kDa linear PEI exerted mechanisms of antiviral activity and the target of its antiviral activity was mainly in the viral adsorption stage. Correspondingly, the study demonstrated that PEI interfered with the virus adsorption stage by electrostatic adsorption. In addition, we found that cationic polymers are a promising novel agent for controlling PRV, and its antiviral mechanism may provide a strategy for the development of antiviral drugs.
Keywords: electrostatic interaction; polyethylenimine; pseudorabies virus; viral adsorption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cathelicidin CATH-B1 Inhibits Pseudorabies Virus Infection via Direct Interaction and TLR4/JNK/IRF3-Mediated Interferon Activation.J Virol. 2023 Jul 27;97(7):e0070623. doi: 10.1128/jvi.00706-23. Epub 2023 Jun 14. J Virol. 2023. PMID: 37314341 Free PMC article.
-
The Valproic Acid Derivative Valpromide Inhibits Pseudorabies Virus Infection in Swine Epithelial and Mouse Neuroblastoma Cell Lines.Viruses. 2021 Dec 15;13(12):2522. doi: 10.3390/v13122522. Viruses. 2021. PMID: 34960791 Free PMC article.
-
Lysimachia christinae polysaccharides dampen pseudorabies viral infection by downregulating adsorption and exert antioxidant activity.Int J Biol Macromol. 2025 May;306(Pt 2):141158. doi: 10.1016/j.ijbiomac.2025.141158. Epub 2025 Feb 16. Int J Biol Macromol. 2025. PMID: 39965698
-
Progress of Research into Novel Drugs and Potential Drug Targets against Porcine Pseudorabies Virus.Viruses. 2022 Aug 11;14(8):1753. doi: 10.3390/v14081753. Viruses. 2022. PMID: 36016377 Free PMC article. Review.
-
A Tug of War: Pseudorabies Virus and Host Antiviral Innate Immunity.Viruses. 2022 Mar 6;14(3):547. doi: 10.3390/v14030547. Viruses. 2022. PMID: 35336954 Free PMC article. Review.
Cited by
-
Alphaherpesvirus in Pets and Livestock.Microorganisms. 2025 Jan 4;13(1):82. doi: 10.3390/microorganisms13010082. Microorganisms. 2025. PMID: 39858850 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 32273015/MOST | National Natural Science Foundation of China (NSFC)
- BK20220114/JST | Jiangsu Natural Science Foundation | Foundation Research Project of Jiangsu Province
- 2018M632399/China Postdoctoral Science Foundation (China Postdoctoral Foundation Project)
- BK20210805/JST | Natural Science Foundation of Jiangsu Province (Jiangsu Natural Science Foundation)
- Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD)
LinkOut - more resources
Full Text Sources

