Time-resolved RNA-seq analysis to unravel the in vivo competence induction by Streptococcus pneumoniae during pneumonia-derived sepsis
- PMID: 38305162
- PMCID: PMC10913500
- DOI: 10.1128/spectrum.03050-23
Time-resolved RNA-seq analysis to unravel the in vivo competence induction by Streptococcus pneumoniae during pneumonia-derived sepsis
Abstract
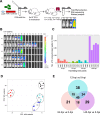
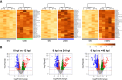
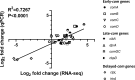
Competence development in Streptococcus pneumoniae (pneumococcus) is tightly intertwined with virulence. In addition to genes encoding genetic transformation machinery, the competence regulon also regulates the expression of allolytic factors, bacteriocins, and cytotoxins. Pneumococcal competence system has been extensively interrogated in vitro where the short transient competent state upregulates the expression of three distinct phases of "early," "late," and "delayed" genes. Recently, we have demonstrated that the pneumococcal competent state develops naturally in mouse models of pneumonia-derived sepsis. To unravel the underlying adaptive mechanisms driving the development of the competent state, we conducted a time-resolved transcriptomic analysis guided by the spatiotemporal live in vivo imaging system of competence induction during pneumonia-derived sepsis. Mouse lungs infected by the serotype 2 strain D39 expressing a competent state-specific reporter gene (D39-ssbB-luc) were subjected to RNA sequencing guided by monitoring the competence development at 0, 12, 24, and, at the moribund state, >40 hours post-infection (hpi). Transcriptomic analysis revealed that the competence-specific gene expression patterns in vivo were distinct from those under in vitro conditions. There was significant upregulation of early, late, and some delayed phase competence-specific genes as early as 12 hpi, suggesting that the pneumococcal competence regulon is important for adaptation to the lung environment. Additionally, members of the histidine triad (pht) gene family were sharply upregulated at 12 hpi followed by a steep decline throughout the rest of the infection cycle, suggesting that Pht proteins participate in the early adaptation to the lung environment. Further analysis revealed that Pht proteins execute a metal ion-dependent regulatory role in competence induction.IMPORTANCEThe induction of pneumococcal competence for genetic transformation has been extensively studied in vitro but poorly understood during lung infection. We utilized a combination of live imaging and RNA sequencing to monitor the development of a competent state during acute pneumonia. Upregulation of competence-specific genes was observed as early as 12 hour post-infection, suggesting that the pneumococcal competence regulon plays an important role in adapting pneumococcus to the stressful lung environment. Among others, we report novel finding that the pneumococcal histidine triad (pht) family of genes participates in the adaptation to the lung environment and regulates pneumococcal competence induction.
Keywords: Pht histidine triad proteins; RNA-seq; Streptococcus pneumoniae; competent state; host adaptation; pneumonia-derived sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Comparative analysis of the Streptococcus pneumoniae competence development in vitro versus in vivo during pneumonia-derived sepsis.Front Microbiol. 2025 Jan 28;16:1540511. doi: 10.3389/fmicb.2025.1540511. eCollection 2025. Front Microbiol. 2025. PMID: 39935640 Free PMC article.
-
Streptococcus pneumoniae Elaborates Persistent and Prolonged Competent State during Pneumonia-Derived Sepsis.Infect Immun. 2020 Mar 23;88(4):e00919-19. doi: 10.1128/IAI.00919-19. Print 2020 Mar 23. Infect Immun. 2020. PMID: 31988172 Free PMC article.
-
Deletion analysis of Streptococcus pneumoniae late competence genes distinguishes virulence determinants that are dependent or independent of competence induction.Mol Microbiol. 2015 Jul;97(1):151-65. doi: 10.1111/mmi.13016. Epub 2015 Apr 24. Mol Microbiol. 2015. PMID: 25846124 Free PMC article.
-
Disentangling competence for genetic transformation and virulence in Streptococcus pneumoniae.Curr Genet. 2016 Feb;62(1):97-103. doi: 10.1007/s00294-015-0520-z. Epub 2015 Sep 24. Curr Genet. 2016. PMID: 26403231 Review.
-
Polyhistidine triad proteins of pathogenic streptococci.Trends Microbiol. 2012 Oct;20(10):485-93. doi: 10.1016/j.tim.2012.06.004. Epub 2012 Jul 20. Trends Microbiol. 2012. PMID: 22819099 Review.
Cited by
-
Comparative analysis of the Streptococcus pneumoniae competence development in vitro versus in vivo during pneumonia-derived sepsis.Front Microbiol. 2025 Jan 28;16:1540511. doi: 10.3389/fmicb.2025.1540511. eCollection 2025. Front Microbiol. 2025. PMID: 39935640 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

