A Randomized, Double-Blind, Phase 3 Safety and Efficacy Study of Ridinilazole Versus Vancomycin for Treatment of Clostridioides difficile Infection: Clinical Outcomes With Microbiome and Metabolome Correlates of Response
- PMID: 38305378
- PMCID: PMC11175683
- DOI: 10.1093/cid/ciad792
A Randomized, Double-Blind, Phase 3 Safety and Efficacy Study of Ridinilazole Versus Vancomycin for Treatment of Clostridioides difficile Infection: Clinical Outcomes With Microbiome and Metabolome Correlates of Response
Abstract
Background: Exposure to antibiotics predisposes to dysbiosis and Clostridioides difficile infection (CDI) that can be severe, recurrent (rCDI), and life-threatening. Nonselective drugs that treat CDI and perpetuate dysbiosis are associated with rCDI, in part due to loss of microbiome-derived secondary bile acid (SBA) production. Ridinilazole is a highly selective drug designed to treat CDI and prevent rCDI.
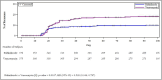
Methods: In this phase 3 superiority trial, adults with CDI, confirmed with a stool toxin test, were randomized to receive 10 days of ridinilazole (200 mg twice daily) or vancomycin (125 mg 4 times daily). The primary endpoint was sustained clinical response (SCR), defined as clinical response and no rCDI through 30 days after end of treatment. Secondary endpoints included rCDI and change in relative abundance of SBAs.
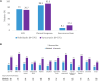
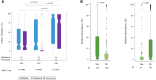
Results: Ridinilazole and vancomycin achieved an SCR rate of 73% versus 70.7%, respectively, a treatment difference of 2.2% (95% CI: -4.2%, 8.6%). Ridinilazole resulted in a 53% reduction in recurrence compared with vancomycin (8.1% vs 17.3%; 95% CI: -14.1%, -4.5%; P = .0002). Subgroup analyses revealed consistent ridinilazole benefit for reduction in rCDI across subgroups. Ridinilazole preserved microbiota diversity, increased SBAs, and did not increase the resistome. Conversely, vancomycin worsened CDI-associated dysbiosis, decreased SBAs, increased Proteobacteria abundance (∼3.5-fold), and increased the resistome.
Conclusions: Although ridinilazole did not meet superiority in SCR, ridinilazole greatly reduced rCDI and preserved microbiome diversity and SBAs compared with vancomycin. These findings suggest that treatment of CDI with ridinilazole results in an earlier recovery of gut microbiome health. Clinical Trials Registration.Ri-CoDIFy 1 and 2: NCT03595553 and NCT03595566.
Keywords: Clostridioides difficile; bile acids; microbiome; ridinilazole; vancomycin.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. K. W. G. received grants paid to his institution from Acurx, Paratek, Cidara, Therapeutics, and Seres Health. P. C. O. received faculty grant/research support from Merck, Sharp and Dohme, Deinove Pharmaceuticals, Summit Pharmaceuticals, Melinta Pharmaceuticals, the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID), Kieberg Foundation, and Napo Pharmaceutical; is a consultant to Napo Pharmaceutical, Ferring Pharmaceutical, Summit Pharmaceutical, SNIPR Biome Company, Haleon, and SNIPR; received payment for speaking from Colegio Mexicano de Medicina Interna; participated on a Scientific Advisory Board for Napo Pharmaceuticals; and has stock or stock options from Moderna, Pfizer, Haleon, Biontech, GSK, Novavax, AstraZeneca, Beam, and Johnson & Johnson. T. L. attended advisory board meetings for Summit, Inc, and received payments for the conduct of clinical trials for Seres Therapeutics, Finch Therapeutics, and Rebiotix/Ferring. E. D. and D. J. were employees of Summit, Inc, and owned stock options in Summit Therapeutics (parent company) at the time of manuscript submission. J. G. M. is a part-time employee and owns stock options in Summit, Inc. E. R. D. has received grants paid to his institution from Theriva Biologics and Ferring and consulting fees from Pfizer, Merck, Seres, Abbott, AstraZeneca, Summit, GSK, and Ferring. M. W. has received consulting fees from AiCuris, Bayer, Crestone, Da Volterra, Deinove, EnteroBiotix, The European Tissue Symposium, Ferring, GSK, Menarini, Merck, Nestlé, Paion, Paratek, Pfizer, Phico Therapeutics, Qpex Biopharma, Seres, Surface Skins, Summit, Tillotts, Vaxxilon/Idorsia, and Vedanta; lecture fees from GSK, Merck, Pfizer, Seres, and Tillotts; and grant support from Almirall, Da Volterra, EnteroBiotix, GSK, Merck, MicroPharm, Nabriva, Paratek, Pfizer, Seres, Summit, The European Tissue Symposium, and Tillotts. J. L., B. Y. C., J. S., L. S., and F. C. are employees of Summit, Inc, and own Summit stock options. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Office of Infectious Disease and HIV/AIDS Policy . Health care-associated infections. Available at: https://www.hhs.gov/oidp/topics/health-care-associated-infections/index..... Accessed 11 January 2022.

