Pulmonary vein narrowing after pulsed field versus thermal ablation
- PMID: 38305503
- PMCID: PMC10875916
- DOI: 10.1093/europace/euae038
Pulmonary vein narrowing after pulsed field versus thermal ablation
Abstract
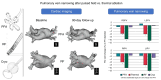
Aims: When it occurs, pulmonary vein (PV) stenosis after atrial fibrillation (AF) ablation is associated with significant morbidity. Even mild-to-moderate PV narrowing may have long-term implications. Unlike thermal ablation energies, such as radiofrequency (RF) or cryothermy, pulsed field ablation (PFA) is a non-thermal modality associated with less fibrotic proliferation. Herein, we compared the effects of PFA vs. thermal ablation on PV narrowing after AF ablation.
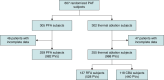
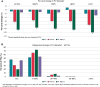
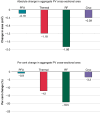
Methods and results: ADVENT was a multi-centre, randomized, single-blind study comparing PFA (pentaspline catheter) with thermal ablation-force-sensing RF or cryoballoon (CB)-to treat drug-refractory paroxysmal AF. Pulmonary vein diameter and aggregate cross-sectional area were obtained by baseline and 3-month imaging. The pre-specified, formally tested, secondary safety endpoint compared a measure of PV narrowing between PFA vs. thermal groups, with superiority defined by posterior probability > 0.975. Among subjects randomized to PFA (n = 305) or thermal ablation (n = 302), 259 PFA and 255 thermal ablation (137 RF and 118 CB) subjects had complete baseline and 3-month PV imaging. No subject had significant (≥70%) PV stenosis. Change in aggregate PV cross-sectional area was less with PFA (-0.9%) than thermal ablation (-12%, posterior probability > 0.999)-primarily driven by the RF sub-cohort (-19.5%) vs. CB sub-cohort (-3.3%). Almost half of all PFA PV diameters did not decrease, but the majority (80%) of RF PVs decreased, regardless of PV anatomic location.
Conclusion: In this first randomized comparison of PFA vs. thermal ablation, PFA resulted in less PV narrowing-thereby underscoring the qualitatively differential and favourable impact of PFA on PV tissue.
Keywords: Atrial fibrillation; Pulmonary vein stenosis; Pulsed field ablation; Randomized controlled trial.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: M.M. is a consultant for Boston Scientific, Biosense Webster, Abbott, Medtronic, Siemens Novartis, Janssen, Boehringer Ingelheim, Pfizer, and SentreHEART/AtriCure and has equity in EPD-Philips (divested), and NewPace Ltd. E.P.G. is a consultant to Farapulse Inc and serves as an unpaid consultant to Boston Scientific Inc and scientific advisory board to Biosense Webster and Adagio Medical, has research support from Biosense-Webster, Adagio Medical, and Abbott, has lecture honoraria from Medtronic, Boston Scientific Inc and Abbott. C.P. is a consultant for Boston Scientific. A.N. is a consultant for Abbott, Baylis, Biotronik, Biosense Webster, Boston Scientific, and Medtronic. W.W. has no relevant disclosures. F.A.C. is a consultant to Boston Scientific and Biosense Webster. S.E.M. is a consultant to Medtronic and Boston Scientific Inc, has research support from Medtronic, Biotronik, Abbott, and CVRx, and has lecture honoraria from Biosense Webster, Medtronic, Boston Scientific Inc, Zoll, and Abbott. D.N.G. is a consultant to Abbott, Baylis, Biotronik, Biosense Webster, Boston Scientific, and Medtronic. J.D.H. has no relevant disclosures. S.K.H. is an employee of Medpace Core Laboratories. C.W.S., A.B.A., E.M.A., and K.M.S. are employees of Boston Scientific. A.S.M. is a consultant to Farapulse Inc and serves as a consultant to Boston Scientific Inc; unrelated to this manuscript, he has also provided statistical consulting and/or Data Safety Monitoring Board services for AtriCure, Abbott, Biosense Webster, and Medtronic. J.W.L. is a consultant to and received equity from Farapulse Inc (now divested) and serves as a consultant to Boston Scientific Inc. V.Y.R. is a Farapulse-Boston Scientific Inc: grant support, consultant, equity (now divested); and unrelated to this manuscript, V.Y.R. also serves as a consultant for and has equity in Ablacon, Acutus Medical, Affera-Medtronic, Apama Medical-Boston Scientific, Anumana, APN Health, AquaHeart, AtaCor, Autonomix, Axon Therapies, Backbeat, BioSig, CardiaCare, CardioNXT/AFTx, Circa Scientific, CoRISMA, Corvia Medical, Dinova-Hangzhou DiNovA EP Technology, East End Medical, EPD-Philips, EP Frontiers, Epix Therapeutics-Medtronic, EpiEP, Eximo, Field Medical, Focused Therapeutics, HRT, Intershunt, Javelin, Kardium, Keystone Heart, LuxMed, MedLumics, Middlepeak, NeuTrace, Nuvera-Biosense Webster, Oracle Health, Restore Medical, Sirona Medical, SoundCath, Valcare; unrelated to this work, V.Y.R. has served as a consultant for Abbott, AtriAN, Biosense Webster, BioTel Heart, Biotronik, Cairdac, CardioFocus, Cardionomic, CoreMap, Fire1, Gore & Associates, Impulse Dynamics, Medtronic, Novartis, Philips, and Pulse Biosciences and has equity in DRS Vascular, Manual Surgical Sciences, NewPace, Nyra Medical, SureCor, and VizaraMed.
Figures


References
-
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jret al. . 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. - PubMed
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. - PubMed
-
- Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. . 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Heart Rhythm 2017;14:e445–94. - PubMed
-
- Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne Jet al. . Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. - PubMed
-
- Writing Committee Members, Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JYet al. . 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol 2024;83:109–279. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

