The neuronal transcription factor MEIS2 is a calpain-2 protease target
- PMID: 38305737
- PMCID: PMC10941658
- DOI: 10.1242/jcs.261482
The neuronal transcription factor MEIS2 is a calpain-2 protease target
Abstract
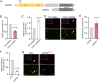
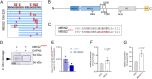
Tight control over transcription factor activity is necessary for a sensible balance between cellular proliferation and differentiation in the embryo and during tissue homeostasis by adult stem cells, but mechanistic details have remained incomplete. The homeodomain transcription factor MEIS2 is an important regulator of neurogenesis in the ventricular-subventricular zone (V-SVZ) adult stem cell niche in mice. We here identify MEIS2 as direct target of the intracellular protease calpain-2 (composed of the catalytic subunit CAPN2 and the regulatory subunit CAPNS1). Phosphorylation at conserved serine and/or threonine residues, or dimerization with PBX1, reduced the sensitivity of MEIS2 towards cleavage by calpain-2. In the adult V-SVZ, calpain-2 activity is high in stem and progenitor cells, but rapidly declines during neuronal differentiation, which is accompanied by increased stability of MEIS2 full-length protein. In accordance with this, blocking calpain-2 activity in stem and progenitor cells, or overexpression of a cleavage-insensitive form of MEIS2, increased the production of neurons, whereas overexpression of a catalytically active CAPN2 reduced it. Collectively, our results support a key role for calpain-2 in controlling the output of adult V-SVZ neural stem and progenitor cells through cleavage of the neuronal fate determinant MEIS2.
Keywords: Adult neurogenesis; Calpain; MEIS homeodomain protein; Stem cell; Subventricular zone; Transcription factor.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures






References
-
- Agoston, Z., Heine, P., Brill, M. S., Grebbin, B. M., Hau, A. C., Kallenborn-Gerhardt, W., Schramm, J., Götz, M. and Schulte, D. (2014). Meis2 is a Pax6 co-factor in neurogenesis and dopaminergic periglomerular fate specification in the adult olfactory bulb. Development 141, 28-38. 10.1242/dev.097295 - DOI - PubMed
-
- Amini, M., Ma, C., Farazifard, R., Zhu, G., Zhang, Y., Vanderluit, J., Zoltewicz, J. S., Hage, F., Savitt, J. M., Lagace, D. C.et al. . (2013). Conditional disruption of calpain in the CNS alters dendrite morphology, impairs LTP, and promotes neuronal survival following injury. J. Neurosci. 33, 5773-5784. 10.1523/JNEUROSCI.4247-12.2013 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

