Transsynaptic Degeneration of Retinal Ganglion Cells Following Lesions to Primary Visual Cortex in Marmosets
- PMID: 38306108
- PMCID: PMC10851175
- DOI: 10.1167/iovs.65.2.4
Transsynaptic Degeneration of Retinal Ganglion Cells Following Lesions to Primary Visual Cortex in Marmosets
Abstract
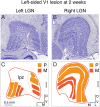
Purpose: A lesion to primary visual cortex (V1) in primates can produce retrograde transneuronal degeneration in the dorsal lateral geniculate nucleus (LGN) and retina. We investigated the effect of age at time of lesion on LGN volume and retinal ganglion cell (RGC) density in marmoset monkeys.
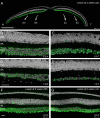
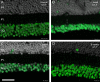
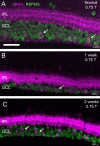
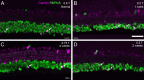
Methods: Retinas and LGNs were obtained about 2 years after a unilateral left-sided V1 lesion as infants (n = 7) or young adult (n = 1). Antibodies against RBPMS were used to label all RGCs, and antibodies against CaMKII or GABAA receptors were used to label nonmidget RGCs. Cell densities were compared in the left and right hemiretina of each eye. The LGNs were stained with the nuclear marker NeuN or for Nissl substance.
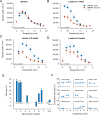
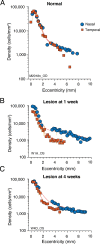
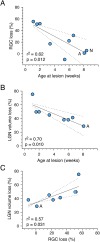
Results: In three animals lesioned within the first 2 postnatal weeks, the proportion of RGCs lost within 5 mm of the fovea was ∼twofold higher than after lesions at 4 or 6 weeks. There was negligible loss in the animal lesioned at 2 years of age. A positive correlation between RGC loss and LGN volume reduction was evident. No loss of CaMKII-positive or GABAA receptor-positive RGCs was apparent within 2 mm of the fovea in any of the retinas investigated.
Conclusions: Susceptibility of marmoset RGCs to transneuronal degeneration is high at birth and declines over the first 6 postnatal weeks. High survival rates of CaMKII and GABAA receptor-positive RGCs implies that widefield and parasol cells are less affected by neonatal cortical lesions than are midget-pathway cells.
Conflict of interest statement
Disclosure:
Figures


Similar articles
-
Retrograde transneuronal degeneration in the retina and lateral geniculate nucleus of the V1-lesioned marmoset monkey.Brain Struct Funct. 2015 Jan;220(1):351-60. doi: 10.1007/s00429-013-0659-7. Epub 2013 Oct 31. Brain Struct Funct. 2015. PMID: 24173617
-
Neuronal degeneration in the dorsal lateral geniculate nucleus following lesions of primary visual cortex: comparison of young adult and geriatric marmoset monkeys.Brain Struct Funct. 2017 Sep;222(7):3283-3293. doi: 10.1007/s00429-017-1404-4. Epub 2017 Mar 22. Brain Struct Funct. 2017. PMID: 28331974
-
Parameters affecting the loss of ganglion cells of the retina following ablations of striate cortex in primates.Vis Neurosci. 1989 Oct;3(4):327-49. doi: 10.1017/s0952523800005514. Vis Neurosci. 1989. PMID: 2487111
-
[Morphological and functional diversity of retinal ganglion cells in the common marmoset].Brain Nerve. 2015 Feb;67(2):193-8. doi: 10.11477/mf.1416200111. Brain Nerve. 2015. PMID: 25681364 Review. Japanese.
-
Molecular determinants of retinal ganglion cell development, survival, and regeneration.Prog Retin Eye Res. 2003 Jul;22(4):483-543. doi: 10.1016/s1350-9462(03)00027-2. Prog Retin Eye Res. 2003. PMID: 12742393 Review.
Cited by
-
Rapid degeneration and neurochemical plasticity of the lateral geniculate nucleus following lesions of the primary visual cortex in marmoset monkeys.Curr Res Neurobiol. 2024 Nov 28;8:100141. doi: 10.1016/j.crneur.2024.100141. eCollection 2025 Jun. Curr Res Neurobiol. 2024. PMID: 39759967 Free PMC article.
-
Spatial Distribution and Morphology of CaMKII-Expressing Amacrine Cells in Marmoset, Macaque, and Human Retina.J Comp Neurol. 2025 Jul;533(7):e70078. doi: 10.1002/cne.70078. J Comp Neurol. 2025. PMID: 40705462 Free PMC article.
References
-
- Cowey A. Atrophy of retinal ganglion cells after removal of striate cortex in a rhesus monkey. Perception . 1974; 3: 257–260. - PubMed
-
- Weller RE, Kaas JH, Wetzel AB.. Evidence for the loss of X-cells of the retina after long-term ablation of visual cortex in monkeys. Brain Res . 1979; 160: 134–138. - PubMed
-
- Cowey A, Stoerig P, Perry VH.. Transneuronal retrograde degeneration of retinal ganglion cells after damage to striate cortex in macaque monkeys: selective loss of P beta cells. Neuroscience . 1989; 29: 65–80. - PubMed
-
- Ptito M, Herbin M, Boire D, Ptito A.. Neural bases of residual vision in hemicorticectomized monkeys. Prog Brain Res . 1996; 112: 385–404. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

