Venous and arterial thrombosis in patients with VEXAS syndrome
- PMID: 38306657
- PMCID: PMC11143532
- DOI: 10.1182/blood.2023022329
Venous and arterial thrombosis in patients with VEXAS syndrome
Abstract
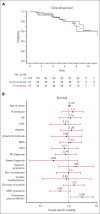
VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome, caused by somatic mutations in UBA1, is an autoinflammatory disorder with diverse systemic manifestations. Thrombosis is a prominent clinical feature of VEXAS syndrome. The risk factors and frequency of thrombosis in VEXAS syndrome are not well described, due to the disease's recent discovery and the paucity of large databases. We evaluated 119 patients with VEXAS syndrome for venous and arterial thrombosis and correlated their presence with clinical outcomes and survival. Thrombosis occurred in 49% of patients, mostly venous thromboembolism (VTE; 41%). Almost two-thirds of VTEs were unprovoked, 41% were recurrent, and 20% occurred despite anticoagulation. The cumulative incidence of VTE was 17% at 1 year from symptom onset and 40% by 5 years. Cardiac and pulmonary inflammatory manifestations were associated with time to VTE. M41L was positively associated specifically with pulmonary embolism by univariate (odds ratio [OR]: 4.58, confidence interval [CI] 1.28-16.21, P = .02) and multivariate (OR: 16.94, CI 1.99-144.3, P = .01) logistic regression. The cumulative incidence of arterial thrombosis was 6% at 1 year and 11% at 5 years. The overall survival of the entire patient cohort at median follow-up time of 4.8 years was 88%, and there was no difference in survival between patients with or without thrombosis (P = .8). Patients with VEXAS syndrome are at high risk of VTE; thromboprophylaxis should administered be in high-risk settings unless strongly contraindicated.
Conflict of interest statement
Conflict-of-interest disclosure: N.S.Y. has a cooperative research and development agreement with Novartis. M.M.P. has research funding from Kura, Stem Line, Epigenetix, and Polaris and is on the advisory board for CTI Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures




Comment in
-
VEXAS syndrome: from a vascular perspective.Blood. 2024 May 23;143(21):2118-2120. doi: 10.1182/blood.2024024161. Blood. 2024. PMID: 38780918 No abstract available.
References
-
- Collins JC, Balanda N, Magaziner SJ, et al. Novel disease-causing mutations in UBA1 reveal disease mechanisms in bone marrow failure and inflammation. Blood. 2022;140(Supplement 1):2914–2915.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

