Validating a minipig model of reversible cerebral demyelination using human diagnostic modalities and electron microscopy
- PMID: 38306899
- PMCID: PMC10850420
- DOI: 10.1016/j.ebiom.2024.104982
Validating a minipig model of reversible cerebral demyelination using human diagnostic modalities and electron microscopy
Abstract
Background: Inflammatory demyelinating diseases of the central nervous system, such as multiple sclerosis, are significant sources of morbidity in young adults despite therapeutic advances. Current murine models of remyelination have limited applicability due to the low white matter content of their brains, which restricts the spatial resolution of diagnostic imaging. Large animal models might be more suitable but pose significant technological, ethical and logistical challenges.
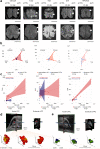
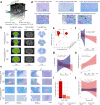
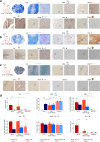
Methods: We induced targeted cerebral demyelinating lesions by serially repeated injections of lysophosphatidylcholine in the minipig brain. Lesions were amenable to follow-up using the same clinical imaging modalities (3T magnetic resonance imaging, 11C-PIB positron emission tomography) and standard histopathology protocols as for human diagnostics (myelin, glia and neuronal cell markers), as well as electron microscopy (EM), to compare against biopsy data from two patients.
Findings: We demonstrate controlled, clinically unapparent, reversible and multimodally trackable brain white matter demyelination in a large animal model. De-/remyelination dynamics were slower than reported for rodent models and paralleled by a degree of secondary axonal pathology. Regression modelling of ultrastructural parameters (g-ratio, axon thickness) predicted EM features of cerebral de- and remyelination in human data.
Interpretation: We validated our minipig model of demyelinating brain diseases by employing human diagnostic tools and comparing it with biopsy data from patients with cerebral demyelination.
Funding: This work was supported by the DFG under Germany's Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy, ID 390857198) and TRR 274/1 2020, 408885537 (projects B03 and Z01).
Keywords: Electromagnetic-guided navigation system; Inflammatory-demyelinating brain disease; In vivo minipig model; Lysophosphatidylcholine; PET/MRI; Scanning electron microscopy.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests CS, SH, BL, EL and TL are part of Ergosurg GmbH, which developed and manufactured the navigation system, the trackable instruments and the robotic system. VMB has received consulting fees from Brainlab. IY has received grants from the German Federal Ministry of Education and Research (BMBF) and the German Research Foundation (DFG), consulting fees from ABX-CRO, Blue Earth Diagnostics and Pentixapharm, honoraria from Piramal, support for attending meeting from the Society of Nuclear Medicine and Molecular Imaging, the European Association of Nuclear Medicine, the Slovenian Neuroscience Association (SiNAPSA) and the International Brain Research Organization, and is a member of the Neuroimaging Committee, European Association of Nuclear Medicine, the Board of Directors, Brain Imaging Council, Society of Nuclear Medicine and Molecular Imaging as well as the Molecular Connectivity Working Group. JK has received consulting fees from Novartis, possesses stock options at Bonescreen GmbH and was supported by the European Research Council, the DFG and the BMBF. TM has received speaker fees from Novartis and Roche as well as travel support from Novartis. BH has received consulting fees from GLG Consulting, Sandoz and Polpharma, possesses issued patents for detection of antibodies against KIR4.1 in a subpopulation of patients with multiple sclerosis, as well as genetic determinants of neutralizing antibodies to interferon, and has participated on Data Safety Monitoring and Advisory Boards for Novartis, Sandoz, Polpharma, Allergycare, TG Therapeutics and Biocon.
Figures



References
-
- Dendrou C.A., Fugger L., Friese M.A. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. - PubMed
-
- Filippi M., Brück W., Chard D.T., et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2019;18:198–210. - PubMed
-
- Plemel J.R., Liu W.-Q., Yong V.W. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov. 2017;16:617–634. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

