Metabolic regulation of the hallmarks of stem cell biology
- PMID: 38306993
- PMCID: PMC10842269
- DOI: 10.1016/j.stem.2024.01.003
Metabolic regulation of the hallmarks of stem cell biology
Abstract
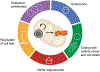
Stem cells perform many different functions, each of which requires specific metabolic adaptations. Over the past decades, studies of pluripotent and tissue stem cells have uncovered a range of metabolic preferences and strategies that correlate with or exert control over specific cell states. This review aims to describe the common themes that emerge from the study of stem cell metabolism: (1) metabolic pathways supporting stem cell proliferation, (2) metabolic pathways maintaining stem cell quiescence, (3) metabolic control of cellular stress responses and cell death, (4) metabolic regulation of stem cell identity, and (5) metabolic requirements of the stem cell niche.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.T.J. and L.W.S.F. are listed as inventors on patent application(s) related to metabolism and cell fate control in embryonic stem cells.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

