Evolution of human H3N2 influenza virus receptor specificity has substantially expanded the receptor-binding domain site
- PMID: 38307019
- PMCID: PMC11057904
- DOI: 10.1016/j.chom.2024.01.003
Evolution of human H3N2 influenza virus receptor specificity has substantially expanded the receptor-binding domain site
Abstract
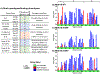
Hemagglutinins (HAs) from human influenza viruses descend from avian progenitors that bind α2-3-linked sialosides and must adapt to glycans with α2-6-linked sialic acids on human airway cells to transmit within the human population. Since their introduction during the 1968 pandemic, H3N2 viruses have evolved over the past five decades to preferentially recognize human α2-6-sialoside receptors that are elongated through addition of poly-LacNAc. We show that more recent H3N2 viruses now make increasingly complex interactions with elongated receptors while continuously selecting for strains maintaining this phenotype. This change in receptor engagement is accompanied by an extension of the traditional receptor-binding site to include residues in key antigenic sites on the surface of HA trimers. These results help explain the propensity for selection of antigenic variants, leading to vaccine mismatching, when H3N2 viruses are propagated in chicken eggs or cells that do not contain such receptors.
Keywords: H3N2; epistasis; glycan; hemagglutinin; host adaptation; human; influenza; receptor; receptor-binding site; sialic acid.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Centers for Disease Control and Prevention (2019). 1918 Pandemic (H1N1 virus) (Centers for Disease Control and Prevention). https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html
-
- Reperant LA, Kuiken T, and Osterhaus AD (2012). Adaptive pathways of zoonotic influenza viruses: from exposure to establishment in humans. Vaccine 30, 4419–4434. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

