Lactoylglutathione promotes inflammatory signaling in macrophages through histone lactoylation
- PMID: 38307385
- PMCID: PMC10869261
- DOI: 10.1016/j.molmet.2024.101888
Lactoylglutathione promotes inflammatory signaling in macrophages through histone lactoylation
Abstract
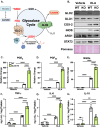
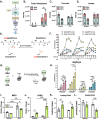
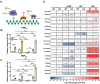
Chronic, systemic inflammation is a pathophysiological manifestation of metabolic disorders. Inflammatory signaling leads to elevated glycolytic flux and a metabolic shift towards aerobic glycolysis and lactate generation. This rise in lactate corresponds with increased generation of lactoylLys modifications on histones, mediating transcriptional responses to inflammatory stimuli. Lactoylation is also generated through a non-enzymatic S-to-N acyltransfer from the glyoxalase cycle intermediate, lactoylglutathione (LGSH). Here, we report a regulatory role for LGSH in mediating histone lactoylation and inflammatory signaling. In the absence of the primary LGSH hydrolase, glyoxalase 2 (GLO2), RAW264.7 macrophages display significant elevations in LGSH and histone lactoylation with a corresponding potentiation of the inflammatory response when exposed to lipopolysaccharides. An analysis of chromatin accessibility shows that lactoylation is associated with more compacted chromatin than acetylation in an unstimulated state; upon stimulation, however, regions of the genome associated with lactoylation become markedly more accessible. Lastly, we demonstrate a spontaneous S-to-S acyltransfer of lactate from LGSH to CoA, yielding lactoyl-CoA. This represents the first known mechanism for the generation of this metabolite. Collectively, these data suggest that LGSH, and not intracellular lactate, is the primary driving factor facilitating histone lactoylation and a major contributor to inflammatory signaling.
Keywords: Glyoxalase; Inflammation; Lactate; Metabolism; Post-translational modification.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures


Update of
-
Lactoylglutathione promotes inflammatory signaling in macrophages.bioRxiv [Preprint]. 2023 Oct 10:2023.10.10.561739. doi: 10.1101/2023.10.10.561739. bioRxiv. 2023. Update in: Mol Metab. 2024 Mar;81:101888. doi: 10.1016/j.molmet.2024.101888. PMID: 37873172 Free PMC article. Updated. Preprint.
References
-
- Wan N., Wang N., Yu S., Zhang H., Tang S., Wang D., et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat Methods. 2022;19(7):854–864. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

