GPRC5D as a novel target for the treatment of multiple myeloma: a narrative review
- PMID: 38307865
- PMCID: PMC10837198
- DOI: 10.1038/s41408-023-00966-9
GPRC5D as a novel target for the treatment of multiple myeloma: a narrative review
Erratum in
-
Correction: GPRC5D as a novel target for the treatment of multiple myeloma: a narrative review.Blood Cancer J. 2024 Mar 6;14(1):40. doi: 10.1038/s41408-024-01018-6. Blood Cancer J. 2024. PMID: 38448422 Free PMC article. No abstract available.
Abstract
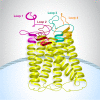
Multiple myeloma is a genetically complex and heterogenous malignancy with a 5-year survival rate of approximately 60%. Despite advances in therapy, patients experience cycles of remission and relapse, with each successive line of therapy associated with poorer outcomes; therefore, therapies with different mechanisms of action against new myeloma antigens are needed. G protein-coupled receptor class C group 5 member D (GPRC5D) has emerged as a novel therapeutic target for the treatment of multiple myeloma. We review the biology and target validation of GPRC5D, and clinical data from early phase trials of GPRC5D-targeting bispecific antibodies, talquetamab and forimtamig, and chimeric antigen receptor T cell (CAR-T) therapies, MCARH109, OriCAR-017, and BMS-986393. In addition to adverse events (AEs) associated with T-cell-redirection therapies irrespective of target, a consistent pattern of dermatologic and oral AEs has been reported across several trials of GPRC5D-targeting bispecific antibodies, as well as rare cerebellar events with CAR-T therapy. Additional studies are needed to understand the underlying mechanisms involved in the development of skin- and oral-related toxicities. We review the strategies that have been used to manage these GPRC5D-related toxicities. Preliminary efficacy data showed overall response rates for GPRC5D-targeting T-cell-redirecting therapies were ≥64%; most responders achieved a very good partial response or better. Pharmacokinetics/pharmacodynamics showed that these therapies led to cytokine release and T-cell activation. In conclusion, results from early phase trials of GPRC5D-targeting T-cell-redirecting agents have shown promising efficacy and manageable safety profiles, including lower infection rates compared with B-cell maturation antigen- and Fc receptor-like protein 5-targeting bispecific antibodies. Further clinical trials, including those investigating GPRC5D-targeting T-cell-redirecting agents in combination with other anti-myeloma therapies and with different treatment modalities, may help to elucidate the future optimal treatment regimen and sequence for patients with multiple myeloma and improve survival outcomes. Video Summary.
© 2024. The Author(s).
Conflict of interest statement
PRO reports a consulting or advisory role with AbbVie, BMS, GlaxoSmithKline, Janssen, Pfizer, and Sanofi; is on the speakers’ bureau for BMS, GlaxoSmithKline, Janssen, and Sanofi; has received travel, accommodations, and expenses from Pfizer; and has received honoraria from AbbVie, Celgene, GlaxoSmithKline, H3 Biomedicine, Janssen, Pfizer, and Sanofi. NWCJvdD has received research funding from Amgen, BMS, Celgene, Cellectis, Janssen, and Novartis; and reports a consulting or advisory role with AbbVie, Adaptive Biotechnologies, Amgen, Bayer, BMS, Celgene, Janssen, Novartis, Pfizer, Roche, Servier, and Takeda. KP is an employee of Janssen Oncology and report stock and other ownership interests in Johnson & Johnson/Janssen. IC is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. DV is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. KG is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. BH is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. JT is an employee of Johnson & Johnson and reports stock and other ownership in Johnson & Johnson; and has received research funding from Janssen Research & Development. TR is an employee of Janssen Oncology and reports stock and other ownership interests in Johnson & Johnson/Janssen. TM is an employee of Janssen Research & Development and reports stock and other ownership interests in Johnson & Johnson. CH is an employee of Janssen Research & Development; reports stock and other ownership interests in Janssen Research & Development; and reports patents, royalties, and other intellectual property in Janssen Research & Development. CK is an employee of Janssen Research & Development; reports stock and other ownership interests in Janssen Research & Development; and has received travel, accommodation, and expenses from Janssen Research & Development. PM has served in a consulting/advisory role and has received honoraria from AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen, Oncopeptides, and Sanofi. NB has served in a consulting/advisory role for AbbVie, Amgen, Bristol Myers Squibb, Forus, Genentech, GlaxoSmithKline, Janssen, Pfizer, Sanofi, and Takeda; has received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Forus, Janssen, Pfizer, and Sanofi; has received research funding from Pfizer; and has served as a member of an IRC and/or steering committee for AbbVie, GlaxoSmithKline, and Janssen. AC reports a consulting or advisory role for AbbVie, Antengene, BMS, Genentech, Genzyme, GlaxoSmithKline, Janssen Oncology, Karyopharm Therapeutics, OncoPeptides, Seagen, Secura Bio, Shattuck Bio, Shattuck Labs, and Takeda; and has received research funding from Amgen, Celgene, Janssen, Pharmacyclics, Seagen, and Takeda.
Figures

References
-
- Cancer stat facts: myeloma. 2023. https://seer.cancer.gov/statfacts/html/mulmy.html.
-
- Globocan 2020 world fact sheet: world. 2020. https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-she...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

