Antibody discovery identifies regulatory mechanisms of protein arginine deiminase 4
- PMID: 38308046
- PMCID: PMC11142921
- DOI: 10.1038/s41589-023-01535-8
Antibody discovery identifies regulatory mechanisms of protein arginine deiminase 4
Abstract
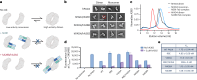
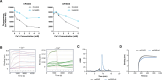
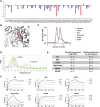
Unlocking the potential of protein arginine deiminase 4 (PAD4) as a drug target for rheumatoid arthritis requires a deeper understanding of its regulation. In this study, we use unbiased antibody selections to identify functional antibodies capable of either activating or inhibiting PAD4 activity. Through cryogenic-electron microscopy, we characterized the structures of these antibodies in complex with PAD4 and revealed insights into their mechanisms of action. Rather than steric occlusion of the substrate-binding catalytic pocket, the antibodies modulate PAD4 activity through interactions with allosteric binding sites adjacent to the catalytic pocket. These binding events lead to either alteration of the active site conformation or the enzyme oligomeric state, resulting in modulation of PAD4 activity. Our study uses antibody engineering to reveal new mechanisms for enzyme regulation and highlights the potential of using PAD4 agonist and antagonist antibodies for studying PAD4-dependency in disease models and future therapeutic development.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: X.Z., S.K., and J.A.W., and the regents of the University of California have filed a provisional patent application related to this project.
Figures












References
Publication types
MeSH terms
Substances
Grants and funding
- R00 EB030587/EB/NIBIB NIH HHS/United States
- (DFS-52-22/Damon Runyon Cancer Research Foundation (Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation)
- R01 GM097316/GM/NIGMS NIH HHS/United States
- K99EB030587/U.S. Department of Health & Human Services | NIH | National Institute of Biomedical Imaging and Bioengineering (NIBIB)
- CA191018/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- DRG-665 2297-17/Damon Runyon Cancer Research Foundation (Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation)
- S10 OD026881/OD/NIH HHS/United States
- T32 HL007731/HL/NHLBI NIH HHS/United States
- R00EB030587/U.S. Department of Health & Human Services | NIH | National Institute of Biomedical Imaging and Bioengineering (NIBIB)
- 1P41CA1962 76/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- GM097316/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- K99 EB030587/EB/NIBIB NIH HHS/United States
- R01 CA191018/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

