A Thermosensitive Bi-Adjuvant Hydrogel Triggers Epitope Spreading to Promote the Anti-Tumor Efficacy of Frameshift Neoantigens
- PMID: 38308098
- PMCID: PMC11005695
- DOI: 10.1002/advs.202306889
A Thermosensitive Bi-Adjuvant Hydrogel Triggers Epitope Spreading to Promote the Anti-Tumor Efficacy of Frameshift Neoantigens
Abstract
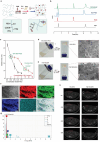
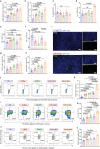
Tumor-specific frameshift mutations encoding peptides (FSPs) are highly immunogenic neoantigens for personalized cancer immunotherapy, while their clinical efficacy is limited by immunosuppressive tumor microenvironment (TME) and self-tolerance. Here, a thermosensitive hydrogel (FSP-RZ-BPH) delivering dual adjuvants R848 (TLR7/8 agonist) + Zn2+ (cGAS-STING agonist) is designed to promote the efficacy of FSPs on murine forestomach cancer (MFC). After peritumoral injection, FSP-RZ-BPH behaves as pH-responsive sustained drug release at sites near the tumor to effectively transform the immunosuppressive TME into an inflammatory type. FSP-RZ-BPH orchestrates innate and adaptive immunity to activate dendritic cells in tumor-draining lymph nodes and increase the number of FSPs-reactive effector memory T cells (TEM) in tumor by 2.9 folds. More importantly, these TEM also exhibit memory responses to nonvaccinated neoantigens on MFC. This epitope spreading effect contributes to reduce self-tolerance to maintain long-lasting anti-tumor immunity. In MFC suppressive model, FSP-RZ-BPH achieves 84.8% tumor inhibition rate and prolongs the survival of tumor-bearing mice with 57.1% complete response rate. As a preventive tumor vaccine, FSP-RZ-BPH can also significantly delay tumor growth. Overall, the work identifies frameshift MFC neoantigens for the first time and demonstrates the thermosensitive bi-adjuvant hydrogel as an effective strategy to boost bystander anti-tumor responses of frameshift neoantigens.
Keywords: TLR7/8 agonist; cGAS‐STING agonist; frameshift neoantigens; thermosensitive hydrogel; tumor immunotherapy.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- a) Poole A., Karuppiah V., Hartt A., Haidar J. N., Moureau S., Dobrzycki T., Hayes C., Rowley C., Dias J., Harper S., Barnbrook K., Hock M., Coles C., Yang W., Aleksic M., Lin A. B., Robinson R., Dukes J. D., Liddy N., Van der Kamp M., Plowman G. D., Vuidepot A., Cole D. K., Whale A. D., Chillakuri C., Nat. Commun. 2022, 13, 5333; - PMC - PubMed
- b) Hsiue E. H., Wright K. M., Douglass J., Hwang M. S., Mog B. J., Pearlman A. H., Paul S., DiNapoli S. R., Konig M. F., Wang Q., Schaefer A., Miller M. S., Skora A. D., Azurmendi P. A., Murphy M. B., Liu Q., Watson E., Li Y., Pardoll D. M., Bettegowda C., Papadopoulos N., Kinzler K. W., Vogelstein B., Gabelli S. B., Zhou S., Science 2021, 371, eabc8697. - PMC - PubMed
-
- a) Smith C. C., Selitsky S. R., Chai S., Armistead P. M., Vincent B. G., Serody J. S., Nat. Rev. Cancer 2019, 19, 465; - PMC - PubMed
- b) Gebert J., Gelincik O., Oezcan‐Wahlbrink M., Marshall J. D., Hernandez‐Sanchez A., Urban K., Long M., Cortes E., Tosti E., Katzenmaier E. M., Song Y., Elsaadi A., Deng N., Vilar E., Fuchs V., Nelius N., Yuan Y. P., Ahadova A., Sei S., Shoemaker R. H., Umar A., Wei L., Liu S., Bork P., Edelmann W., von Knebel Doeberitz M., Lipkin S. M., Kloor M., Gastroenterology 2021, 161, 1288; - PMC - PubMed
- c) Kloor M., Reuschenbach M., Pauligk C., Karbach J., Rafiyan M. R., Al‐Batran S. E., Tariverdian M., Jager E., von Knebel Doeberitz M., Clin. Cancer Res. 2020, 26, 4503. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
