A Single-Atom Manganese Nanozyme Mn-N/C Promotes Anti-Tumor Immune Response via Eliciting Type I Interferon Signaling
- PMID: 38308189
- PMCID: PMC11005736
- DOI: 10.1002/advs.202305979
A Single-Atom Manganese Nanozyme Mn-N/C Promotes Anti-Tumor Immune Response via Eliciting Type I Interferon Signaling
Abstract
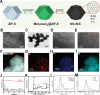
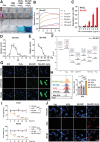
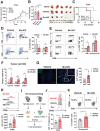
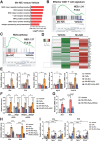
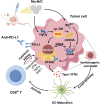
Tumor microenvironment (TME)-induced nanocatalytic therapy is a promising strategy for cancer treatment, but the low catalytic efficiency limits its therapeutic efficacy. Single-atom catalysts (SACs) are a new type of nanozyme with incredible catalytic efficiency. Here, a single-atom manganese (Mn)-N/C nanozyme is constructed. Mn-N/C catalyzes the conversion of cellular H2O2 to ∙OH through a Fenton-like reaction and enables the sufficient generation of reactive oxygen species (ROS), which induces immunogenic cell death (ICD) of tumor cells and significantly promotes CD8+T anti-tumor immunity. Moreover, RNA sequencing analysis reveals that Mn-N/C treatment activates type I interferon (IFN) signaling, which is critical for Mn-N/C-mediated anti-tumor immune response. Mechanistically, the release of cytosolic DNA and Mn2+ triggered by Mn-N/C collectively activates the cGAS-STING pathway, subsequently stimulating type I IFN induction. A highly efficient single-atom nanozyme, Mn-N/C, which enhances anti-tumor immune response and exhibits synergistic therapeutic effects when combined with the anti-PD-L1 blockade, is proposed.
Keywords: ROS; anti‐tumor immunity; nanomedicine; single‐atom nanozyme; type I interferon.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

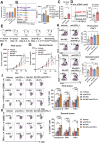
Similar articles
-
Stimuli-Responsive Double Single-Atom Catalysts for Parallel Catalytic Therapy.Pharmaceutics. 2023 Apr 11;15(4):1217. doi: 10.3390/pharmaceutics15041217. Pharmaceutics. 2023. PMID: 37111702 Free PMC article.
-
Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy.Cell Res. 2020 Nov;30(11):966-979. doi: 10.1038/s41422-020-00395-4. Epub 2020 Aug 24. Cell Res. 2020. PMID: 32839553 Free PMC article. Clinical Trial.
-
Stimuli-Responsive Manganese Single-Atom Nanozyme for Tumor Therapy via Integrated Cascade Reactions.Angew Chem Int Ed Engl. 2021 Apr 19;60(17):9480-9488. doi: 10.1002/anie.202017152. Epub 2021 Mar 11. Angew Chem Int Ed Engl. 2021. PMID: 33543825
-
Advances of MnO2 nanomaterials as novel agonists for the development of cGAS-STING-mediated therapeutics.Front Immunol. 2023 Apr 19;14:1156239. doi: 10.3389/fimmu.2023.1156239. eCollection 2023. Front Immunol. 2023. PMID: 37153576 Free PMC article. Review.
-
Role of micronucleus-activated cGAS-STING signaling in antitumor immunity.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jan 26;53(1):25-34. doi: 10.3724/zdxbyxb-2023-0485. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38273467 Free PMC article. Review. Chinese, English.
Cited by
-
MnGA with multiple enzyme-like properties for acute wound healing by reducing oxidative stress and modulating signaling pathways.Mater Today Bio. 2024 Dec 27;30:101435. doi: 10.1016/j.mtbio.2024.101435. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39850242 Free PMC article.
-
Mechanisms and Applications of Manganese-Based Nanomaterials in Tumor Diagnosis and Therapy.Biomater Res. 2025 Feb 28;29:0158. doi: 10.34133/bmr.0158. eCollection 2025. Biomater Res. 2025. PMID: 40026879 Free PMC article. Review.
-
Nano drug delivery systems for advanced immune checkpoint blockade therapy.Theranostics. 2025 Apr 13;15(11):5440-5480. doi: 10.7150/thno.112475. eCollection 2025. Theranostics. 2025. PMID: 40303342 Free PMC article. Review.
-
Tumor Microenvironment-Driven Structural Transformation of Vanadium-Based MXenzymes to Amplify Oxidative Stress for Multimodal Tumor Therapy.Adv Sci (Weinh). 2025 Mar;12(11):e2408998. doi: 10.1002/advs.202408998. Epub 2025 Jan 23. Adv Sci (Weinh). 2025. PMID: 39853632 Free PMC article.
-
Improvement of the Anticancer Efficacy of PD-1/PD-L1 Blockade: Advances in Molecular Mechanisms and Therapeutic Strategies.MedComm (2020). 2025 Jul 15;6(8):e70274. doi: 10.1002/mco2.70274. eCollection 2025 Aug. MedComm (2020). 2025. PMID: 40672434 Free PMC article. Review.
References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., CA Cancer J. Clin. 2021, 71, 209. - PubMed
-
- a) Kim T. K., Vandsemb E. N., Herbst R. S., Chen L., Nat. Rev. Drug Discov. 2022, 21, 529; - PubMed
- b) Sharma P., Goswami S., Raychaudhuri D., Siddiqui B. A., Singh P., Nagarajan A., Liu J., Subudhi S. K., Poon C., Gant K. L., Herbrich S. M., Anandhan S., Islam S., Amit M., Anandappa G., Allison J. P., Cell 2023, 186, 1652. - PubMed
MeSH terms
Substances
Grants and funding
- 82103518/National Natural Science Foundation of China
- 82272997/National Natural Science Foundation of China
- 2020JCPT02/Fundamental Research Funds for the Central Universities
- 22277072/Fundamental Research Funds for the Central Universities
- 22107065/Fundamental Research Funds for the Central Universities
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
