Spatiotemporal Controllable Sono-Nanovaccines Driven by Free-Field Based Whole-Body Ultrasound for Personalized Cancer Therapy
- PMID: 38308196
- PMCID: PMC11005707
- DOI: 10.1002/advs.202307920
Spatiotemporal Controllable Sono-Nanovaccines Driven by Free-Field Based Whole-Body Ultrasound for Personalized Cancer Therapy
Abstract
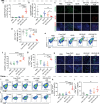
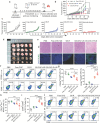
Therapeutic cancer vaccines fail to produce satisfactory outcomes against solid tumors since vaccine-induced anti-tumor immunity is significantly hampered by immunosuppression. Generating an in situ cancer vaccine targeting immunological cold tumor microenvironment (TME) appears attractive. Here, a type of free-field based whole-body ultrasound (US)-driven nanovaccines are constructed, named G5-CHC-R, by conjugating the sonosensitizer, Chenghai chlorin (CHC) and the immunomodulator, resiquimod (R848) on top of a super small-sized dendrimeric nanoscaffold. Once entering tumors, R848 can be cleaved from a hypoxia-sensitive linker, thus modifying the TME via converting macrophage phenotypes. The animals bearing orthotopic pancreatic cancer with intestinal metastasis and breast cancer with lung metastasis are treated with G5-CHC-R under a free-field based whole-body US system. Benefit from the deep penetration capacity and highly spatiotemporal selectiveness, G5-CHC-R triggered by US represented a superior alternative for noninvasive irradiation of deep-seated tumors and magnification of local immune responses via driving mass release of tumor antigens and "cold-warm-hot" three-state transformation of TME. In addition to irradiating primary tumors, a robust adaptive anti-tumor immunity is potentiated, leading to successful induction of systemic tumor suppression. The sono-nanovaccines with good biocompatibility posed wide applicability to a broad spectrum of tumors, revealing immeasurable potential for translational research in oncology.
Keywords: nanovaccines; tumor associated macrophages; tumor microenvironment modulation; ultrasound‐driven; whole‐body sonodynamic therapy.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Monophosphoryl lipid A-assembled nanovaccines enhance tumor immunotherapy.Acta Biomater. 2023 Nov;171:482-494. doi: 10.1016/j.actbio.2023.09.013. Epub 2023 Sep 13. Acta Biomater. 2023. PMID: 37708924
-
In Situ Cocktail Nanovaccine for Cancer Immunotherapy.Adv Sci (Weinh). 2023 Nov;10(31):e2207697. doi: 10.1002/advs.202207697. Epub 2023 Sep 22. Adv Sci (Weinh). 2023. PMID: 37740439 Free PMC article.
-
Nanovaccines for cancer immunotherapy: Focusing on complex formation between adjuvant and antigen.Int Immunopharmacol. 2023 Apr;117:109887. doi: 10.1016/j.intimp.2023.109887. Epub 2023 Feb 24. Int Immunopharmacol. 2023. PMID: 36841155 Review.
-
Self-adjuvanting cancer nanovaccines.J Nanobiotechnology. 2022 Jul 26;20(1):345. doi: 10.1186/s12951-022-01545-z. J Nanobiotechnology. 2022. PMID: 35883176 Free PMC article. Review.
-
Advances in Cancer Nanovaccines: Harnessing Nanotechnology for Broadening Cancer Immune Response.ChemMedChem. 2023 Jul 3;18(13):e202200673. doi: 10.1002/cmdc.202200673. Epub 2023 May 2. ChemMedChem. 2023. PMID: 37088719 Review.
Cited by
-
Nanosensitizer-assisted sonodynamic therapy for breast cancer.J Nanobiotechnology. 2025 Apr 7;23(1):281. doi: 10.1186/s12951-025-03311-3. J Nanobiotechnology. 2025. PMID: 40197318 Free PMC article. Review.
-
Progress in application of nanomedicines for enhancing cancer sono-immunotherapy.Ultrason Sonochem. 2024 Dec;111:107105. doi: 10.1016/j.ultsonch.2024.107105. Epub 2024 Oct 15. Ultrason Sonochem. 2024. PMID: 39427436 Free PMC article. Review.
-
Nano-Oncologic Vaccine for Boosting Cancer Immunotherapy: The Horizons in Cancer Treatment.Nanomaterials (Basel). 2025 Jan 16;15(2):122. doi: 10.3390/nano15020122. Nanomaterials (Basel). 2025. PMID: 39852737 Free PMC article. Review.
References
-
- a) Farkona S., Diamandis E. P., Blasutig I. M., BMC Med. 2016, 14, 73; - PMC - PubMed
- b) Waldman A. D., Fritz J. M., Lenardo M. J., Nat. Rev. Immunol. 2020, 20, 651; - PMC - PubMed
- c) Peterson C., Denlinger N., Yang Y., Cancers 2022, 14, 3972; - PMC - PubMed
- d) Kumar A., Swain C. A., Shevde L. A., Cancer Metastasis Rev. 2021, 40, 549. - PubMed
-
- a) Ugel S., De Sanctis F., Mandruzzato S., Bronte V., J. Clin. Invest. 2015, 125, 3365; - PMC - PubMed
- b) Mantovani A., Allavena P., Marchesi F., Garlanda C., Nat. Rev. Drug Discov. 2022, 21, 799; - PMC - PubMed
- c) Gabrilovich D. I., Nagaraj S., Nat. Rev. Immunol. 2009, 9, 162; - PMC - PubMed
- d) Barry S. T., Gabrilovich D. I., Sansom O. J., Campbell A. D., Morton J. P., Nat. Rev. Cancer 2023, 23, 216. - PubMed
-
- Saxena M., van der Burg S. H., Melief C. J. M., Bhardwaj N., Nat. Rev. Cancer 2021, 21, 360. - PubMed
-
- a) Lin M. J., Svensson‐Arvelund J., Lubitz G. S., Marabelle A., Melero I., Brown B. D., Brody J. D., Nat. Cancer 2022, 3, 911; - PubMed
- b) Hu Z., Ott P. A., Wu C. J., Nat. Rev. Immunol. 2018, 18, 168; - PMC - PubMed
- c) Shao K., Singha S., Clemente‐Casares X., Tsai S., Yang Y., Santamaria P., ACS Nano 2015, 9, 16; - PubMed
- d) Singha S., Shao K., Ellestad K. K., Yang Y., Santamaria P., ACS Nano 2018, 12, 10621; - PubMed
- e) Singha S., Shao K., Yang Y., Clemente‐Casares X., Sole P., Clemente A., Blanco J., Dai Q., Song F., Liu S. W., Yamanouchi J., Umeshappa C. S., Nanjundappa R. H., Detampel P., Amrein M., Fandos C., Tanguay R., Newbigging S., Serra P., Khadra A., Chan W. C. W., Santamaria P., Nat. Nanotechnol. 2017, 12, 701. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
