Targeting Grancalcin Accelerates Wound Healing by Improving Angiogenesis in Diabetes
- PMID: 38308197
- PMCID: PMC11005700
- DOI: 10.1002/advs.202305856
Targeting Grancalcin Accelerates Wound Healing by Improving Angiogenesis in Diabetes
Abstract
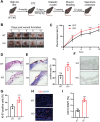
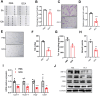
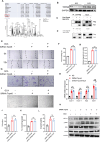
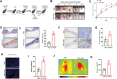
Chronic diabetic wounds are a serious complication of diabetes and often result in limb amputations and confer high mortality rates. The proinflammatory secretome in the wound perpetuates defective neovascularization and contributes to dysregulated tissue repair. This study aims to design a gelatin methacrylamide (GelMA) hydrogel to sustained the release of grancalcin-neutralizing antibody (GCA-NAb) and evaluate it as a potential scaffold to promote diabetic wound healing. Results show that the expression of grancalcin(GCA), a protein secreted by bone marrow-derived immune cells, is elevated in the wound sites of individuals and animals with diabetic ulcers. Genetic inhibition of grancalcin expression accelerates vascularization and healing in an animal model. Mechanistic studies show that grancalcin binds to transient receptor potential melastatin 8(TRPM8) and partially inactivates its downstream signaling pathways, thereby impairing angiogenesis in vitro and ex vivo. Systemic or topical administration of a GCA-NAb accelerate wound repair in mice with diabetes. The data suggest that GCA is a potential therapeutic target for the treatment of diabetic ulcers.
Keywords: angiogenesis; bone marrow‐derived cell; diabetic wound; grancalcin‐neutralizing antibody; hydrogel.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Armstrong D. G., Boulton A. J. M., Bus S. A., N. Engl. J. Med. 2017, 376, 2367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 92149306/National Natural Science Foundation of China
- 82120108009/National Natural Science Foundation of China
- 82072170:81930022/National Natural Science Foundation of China
- 82372512/National Natural Science Foundation of China
- 82100900/National Natural Science Foundation of China
- 2022YFC3601903/National Key Research and Development Program of China
- 2019YFA0110503/National Key Research and Development Program of China
- 2019YFA0110501/National Key Research and Development Program of China
- 2022WK2010/Key field Research and Development Program of Hunan province
- 2021JJ31086/Science Fund for Distinguished Young Scholars of Hunan Province
- 22QA1411700/Shanghai Rising-Star Program
- Youth Medical Talents-Specialist Program
- Changhong Talent Plan of Changhai Hospital
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
