Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer's disease
- PMID: 38308368
- PMCID: PMC10837901
- DOI: 10.1186/s12974-024-03031-9
Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer's disease
Abstract
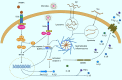
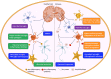
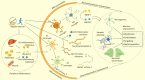
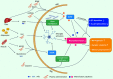
Neuroinflammation is a pathological hallmark of Alzheimer's disease (AD), characterized by the stimulation of resident immune cells of the brain and the penetration of peripheral immune cells. These inflammatory processes facilitate the deposition of amyloid-beta (Aβ) plaques and the abnormal hyperphosphorylation of tau protein. Managing neuroinflammation to restore immune homeostasis and decrease neuronal damage is a therapeutic approach for AD. One way to achieve this is through exercise, which can improve brain function and protect against neuroinflammation, oxidative stress, and synaptic dysfunction in AD models. The neuroprotective impact of exercise is regulated by various molecular factors that can be activated in the same way as exercise by the administration of their mimetics. Recent evidence has proven some exercise mimetics effective in alleviating neuroinflammation and AD, and, additionally, they are a helpful alternative option for patients who are unable to perform regular physical exercise to manage neurodegenerative disorders. This review focuses on the current state of knowledge on exercise mimetics, including their efficacy, regulatory mechanisms, progress, challenges, limitations, and future guidance for their application in AD therapy.
© 2024. The Author(s).
Conflict of interest statement
The author declares that there are no competing interests.
Figures
Similar articles
-
Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair: emerging therapeutic modality for Alzheimer's disease.Mol Neurodegener. 2016 Jul 11;11(1):50. doi: 10.1186/s13024-016-0119-y. Mol Neurodegener. 2016. PMID: 27400746 Free PMC article. Review.
-
Platycodon grandiflorum root extract inhibits Aβ deposition by breaking the vicious circle linking oxidative stress and neuroinflammation in Alzheimer's disease.Biomed Pharmacother. 2024 Aug;177:117090. doi: 10.1016/j.biopha.2024.117090. Epub 2024 Jul 4. Biomed Pharmacother. 2024. PMID: 38968796
-
Design, current states, and challenges of nanomaterials in anti-neuroinflammation: A perspective on Alzheimer's disease.Ageing Res Rev. 2025 Mar;105:102669. doi: 10.1016/j.arr.2025.102669. Epub 2025 Jan 27. Ageing Res Rev. 2025. PMID: 39864562 Review.
-
The neuroprotective N-terminal amyloid-β core hexapeptide reverses reactive gliosis and gliotoxicity in Alzheimer's disease pathology models.J Neuroinflammation. 2023 May 27;20(1):129. doi: 10.1186/s12974-023-02807-9. J Neuroinflammation. 2023. PMID: 37245024 Free PMC article.
-
SLOH, a carbazole-based fluorophore, mitigates neuropathology and behavioral impairment in the triple-transgenic mouse model of Alzheimer's disease.Neuropharmacology. 2018 Mar 15;131:351-363. doi: 10.1016/j.neuropharm.2018.01.003. Epub 2018 Jan 5. Neuropharmacology. 2018. PMID: 29309769
Cited by
-
Platycodin D and voluntary running synergistically ameliorate memory deficits in 5 × FAD mice via mediating neuromodulation and neuroinflammation.Front Aging Neurosci. 2024 Sep 25;16:1451766. doi: 10.3389/fnagi.2024.1451766. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39385832 Free PMC article.
-
Meta-Analysis of Exercise Effects on Cognition in Persons with Parkinson's Disease.NeuroSci. 2025 May 23;6(2):46. doi: 10.3390/neurosci6020046. NeuroSci. 2025. PMID: 40559207 Free PMC article.
-
Molecular Network Analysis and Effector Gene Prioritization of Endurance-Training-Influenced Modulation of Cardiac Aging.Genes (Basel). 2025 Jul 11;16(7):814. doi: 10.3390/genes16070814. Genes (Basel). 2025. PMID: 40725470 Free PMC article.
-
Can exercise benefits be harnessed with drugs? A new way to combat neurodegenerative diseases by boosting neurogenesis.Transl Neurodegener. 2024 Jul 25;13(1):36. doi: 10.1186/s40035-024-00428-7. Transl Neurodegener. 2024. PMID: 39049102 Free PMC article. Review.
-
Exercise-Induced cytokines, diet, and inflammation and their role in adipose tissue metabolism.Health Sci Rep. 2024 Aug 31;7(9):e70034. doi: 10.1002/hsr2.70034. eCollection 2024 Sep. Health Sci Rep. 2024. PMID: 39221051 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

