The skin microbiome in pediatric atopic dermatitis and food allergy
- PMID: 38308490
- PMCID: PMC11142881
- DOI: 10.1111/all.16044
The skin microbiome in pediatric atopic dermatitis and food allergy
Abstract
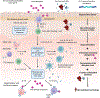
The skin microbiome is an extensive community of bacteria, fungi, mites, viruses and archaea colonizing the skin. Fluctuations in the composition of the skin microbiome have been observed in atopic dermatitis (AD) and food allergy (FA), particularly in early life, established disease, and associated with therapeutics. However, AD is a multifactorial disease characterized by skin barrier aberrations modulated by genetics, immunology, and environmental influences, thus the skin microbiome is not the sole feature of this disease. Future research should focus on mechanistic understanding of how early-life skin microbial shifts may influence AD and FA onset, to guide potential early intervention strategies or as microbial biomarkers to identify high-risk infants who may benefit from possible microbiome-based biotherapeutic strategies. Harnessing skin microbes as AD biotherapeutics is an emerging field, but more work is needed to investigate whether this approach can lead to sustained clinical responses.
Keywords: Staphylococcus aureus; atopic dermatitis; food allergy; microbiota; skin microbiome.
© 2024 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
Conflict of Interest Statement
The authors declared no conflicts of interest
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

