Further investigations on the influence of protein phosphatases on the signaling of muscarinic receptors in the atria of mouse hearts
- PMID: 38308688
- PMCID: PMC11329414
- DOI: 10.1007/s00210-024-02973-4
Further investigations on the influence of protein phosphatases on the signaling of muscarinic receptors in the atria of mouse hearts
Abstract
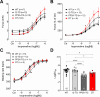
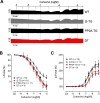
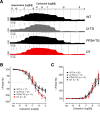
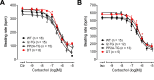
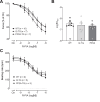
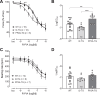
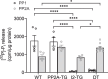
The vagal regulation of cardiac function involves acetylcholine (ACh) receptor activation followed by negative chronotropic and negative as well as positive inotropic effects. The resulting signaling pathways may include Gi/o protein-coupled reduction in adenylyl cyclase (AC) activity, direct Gi/o protein-coupled activation of ACh-activated potassium current (IKACh), inhibition of L-type calcium ion channels, and/or the activation of protein phosphatases. Here, we studied the role of the protein phosphatases 1 (PP1) and 2A (PP2A) for muscarinic receptor signaling in isolated atrial preparations of transgenic mice with cardiomyocyte-specific overexpression of either the catalytic subunit of PP2A (PP2A-TG) or the inhibitor-2 (I2) of PP1 (I2-TG) or in double transgenic mice overexpressing both PP2A and I2 (DT). In mouse left atrial preparations, carbachol (CCh), cumulatively applied (1 nM-10 µM), exerted at low concentrations a negative inotropic effect followed by a positive inotropic effect at higher concentrations. This biphasic effect was noted with CCh alone as well as when CCh was added after β-adrenergic pre-stimulation with isoprenaline (1 µM). Whereas the response to stimulation of β-adrenoceptors or adenosine receptors (used as controls) was changed in PP2A-TG, the response to CCh was unaffected in atrial preparations from all transgenic models studied here. Therefore, the present data tentatively indicate that neither PP2A nor PP1, but possibly other protein phosphatases, is involved in the muscarinic receptor-induced inotropic and chronotropic effects in the mouse heart.
Keywords: Heart; Inhibitor-2; Muscarinic receptor; PP1; PP2A; Protein phosphorylation; Transgenic mice.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Genetic disruption of G proteins, G(i2)alpha or G(o)alpha, does not abolish inotropic and chronotropic effects of stimulating muscarinic cholinoceptors in atrium.Br J Pharmacol. 2009 Nov;158(6):1557-64. doi: 10.1111/j.1476-5381.2009.00441.x. Br J Pharmacol. 2009. PMID: 19906118 Free PMC article.
-
Cardiac function is regulated by B56α-mediated targeting of protein phosphatase 2A (PP2A) to contractile relevant substrates.J Biol Chem. 2014 Dec 5;289(49):33862-73. doi: 10.1074/jbc.M114.598938. Epub 2014 Oct 15. J Biol Chem. 2014. PMID: 25320082 Free PMC article.
-
Cardiostimulant and cardiodepressant effects through overexpressed human beta2-adrenoceptors in murine heart: regional differences and functional role of beta1-adrenoceptors.Naunyn Schmiedebergs Arch Pharmacol. 2003 Apr;367(4):380-90. doi: 10.1007/s00210-002-0681-4. Epub 2003 Mar 4. Naunyn Schmiedebergs Arch Pharmacol. 2003. PMID: 12690430
-
Regulation of phospholamban and troponin-I phosphorylation in the intact rat cardiomyocytes by adrenergic and cholinergic stimuli: roles of cyclic nucleotides, calcium, protein kinases and phosphatases and depolarization.Mol Cell Biochem. 1995 Aug-Sep;149-150:103-26. doi: 10.1007/BF01076569. Mol Cell Biochem. 1995. PMID: 8569720 Review.
-
[Muscarinic modulation of cardiac activity].J Soc Biol. 1999;193(6):469-80. J Soc Biol. 1999. PMID: 10783705 Review. French.
Cited by
-
The Role of Muscarinic Acetylcholine Receptor M3 in Cardiovascular Diseases.Int J Mol Sci. 2024 Jul 10;25(14):7560. doi: 10.3390/ijms25147560. Int J Mol Sci. 2024. PMID: 39062802 Free PMC article. Review.
-
Research progress in myocardial function and diseases related to muscarinic acetylcholine receptor (Review).Int J Mol Med. 2025 Jun;55(6):86. doi: 10.3892/ijmm.2025.5527. Epub 2025 Apr 4. Int J Mol Med. 2025. PMID: 40183403 Free PMC article. Review.
-
Inhibition of protein phosphatases attenuates A1-adenosine receptor-stimulation induced negative inotropic effects of cAMP-increasing agents in the isolated human atrium.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):9125-9138. doi: 10.1007/s00210-025-03854-0. Epub 2025 Feb 5. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39907786 Free PMC article.
References
-
- Bognar IT, Beinhauer B, Kann P, Fuder H (1990) Different muscarinic receptors mediate autoinhibition of acetylcholine release and vagally-induced vasoconstriction in the rat isolated perfused heart. Naunyn Schmiedebergs Arch Pharmacol 341:279–287. 10.1007/BF00180652 10.1007/BF00180652 - DOI - PubMed
-
- Boknik P, Grote-Wessels S, Barteska G, Jiang M, Müller FU, Schmitz W, Neumann J, Birnbaumer L (2009) Genetic disruption of G proteins, G(i2)alpha or G(o)alpha, does not abolish inotropic and chronotropic effects of stimulating muscarinic cholinoceptors in atrium. Br J Pharmacol 158:1557–1564. 10.1111/j.1476-5381.2009.00441.x 10.1111/j.1476-5381.2009.00441.x - DOI - PMC - PubMed
-
- Brodde OE, Michel MC (1999) Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev 51:651–690 - PubMed
-
- Brüchert N, Mavila N, Boknik P, Baba HA, Fabritz L, Gergs U, Kirchhefer U, Kirchhof P, Matus M, Schmitz W, Depaoli-Roach AA, Neumann J (2008) Inhibitor-2 prevents protein phosphatase 1-induced cardiac hypertrophy and mortality. Am J Physiol Heart Circ Physiol 295:H1539–H1546. 10.1152/ajpheart.00515.2008 10.1152/ajpheart.00515.2008 - DOI - PMC - PubMed
-
- Cheng G, Takahashi M, Shunmugavel A, Wallenborn JG, Depaoli-Roach AA, Gergs U, Neumann J, Kuppuswamy D, Menick DR, Cooper G (2010) Basis for MAP4 dephosphorylation-related microtubule network densification in pressure overload cardiac hypertrophy. J Biol Chem 285:38125–38140. 10.1074/jbc.M110.148650 10.1074/jbc.M110.148650 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

