Bemarituzumab as first-line treatment for locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma: final analysis of the randomized phase 2 FIGHT trial
- PMID: 38308771
- PMCID: PMC11016503
- DOI: 10.1007/s10120-024-01466-w
Bemarituzumab as first-line treatment for locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma: final analysis of the randomized phase 2 FIGHT trial
Abstract
Background: We report the final results of the randomized phase 2 FIGHT trial that evaluated bemarituzumab, a humanized monoclonal antibody selective for fibroblast growth factor receptor 2b (FGFR2b), plus mFOLFOX6 in patients with FGFR2b-positive (2 + /3 + membranous staining by immunohistochemistry), HER-2-negative gastric or gastroesophageal junction cancer (GC).
Methods: Patients received bemarituzumab (15 mg/kg) or placebo once every 2 weeks with an additional bemarituzumab (7.5 mg/kg) or placebo dose on cycle 1 day 8. All patients received mFOLFOX6. The primary endpoint was investigator-assessed progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate, and safety. Efficacy was evaluated after a minimum follow-up of 24 months.
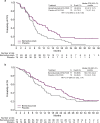
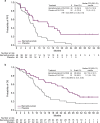
Results: In the bemarituzumab-mFOLFOX6 (N = 77) and placebo-mFOLFOX6 (N = 78) arms, respectively, 59.7% and 66.7% of patients were FGFR2b-positive in ≥ 10% of tumor cells. The median PFS (95% confidence interval [CI]) was 9.5 months (7.3-13.7) with bemarituzumab-mFOLFOX6 and 7.4 months (5.7-8.4) with placebo-mFOLFOX6 (hazard ratio [HR], 0.72; 95% CI 0.49-1.08); median OS (95% CI) was 19.2 (13.6-24.2) and 13.5 (9.3-15.9) months, respectively (HR 0.77; 95% CI 0.52-1.14). Observed efficacy in FGFR2b-positive GC in ≥ 10% of tumor cells was: PFS: HR 0.43 (95% CI 0.26-0.73); OS: HR 0.52 (95% CI 0.31-0.85). No new safety findings were reported.
Conclusions: In FGFR2b-positive advanced GC, the combination of bemarituzumab-mFOLFOX6 led to numerically longer median PFS and OS compared with mFOLFOX6 alone. Efficacy was more pronounced with FGFR2b overexpression in ≥ 10% of tumor cells. Confirmatory phase 3 trials are ongoing (NCT05052801, NCT05111626).
Clinical trial registration: NCT03694522.
Keywords: Bemarituzumab; FGFR2b; Gastric cancer; Targeted therapy; mFOLFOX6.
© 2024. The Author(s).
Conflict of interest statement
Zev A. Wainberg: Honoraria (self): Amgen, Arcus, AstraZeneca, Daiichi, Bayer, Bristol Myers Squibb (BMS), Merck, Ipsen, Gilead, Arcus, Astellas, Seagen, Novartis; Advisory/Consultancy: Amgen, Arcus, AstraZeneca, Daiichi, Bayer, BMS, Merck, Ipsen, Novartis, Gilead, Arcus, Astellas, Seagen; Research grant/funding to institution: Amgen, AstraZeneca, Daiichi, Bayer, BMS, Merck, Ipsen, Five Prime, Gilead, Arcus, Astellas, Molecular Templates, Roche/Genentech, Array/Pfizer. Yoon-Koo Kang: Consulting fees (self): Amgen, Novartis, Roche, Daehwa, Zymeworks, Blueprint, Surface Oncology, ALX Oncology, MacroGenics, BMS, Merck, LISCure. Keun-Wook Lee: All support for the present manuscript: Five Prime Therapeutics; Grants or contracts from any entity: All to institution for conducting clinical trials—AstraZeneca, Ono Pharmaceutical, Merck Sharp and Dohme, Merck KGaA, Roche, Pfizer, BeiGene, Leap Therapeutics, ALX Oncology, Zymeworks, Astellas, MacroGenics, Amgen, Seagen, Bolt Therapeutics, Trishula Therapeutics, Oncologie, Pharmacyclics, MedPacto, Green Cross Corp, ABL Bio, Y-Biologics, Daiichi Sankyo, Taiho Pharmaceutical, InventisBio, Elevar Therapeutics, Metafines, Idience, Genome & Company, Exelixis; Honoraria for lectures: Ono Pharmaceutical, Boryung, Daiichi Sankyo, Astellas, Sanofi-Aventis; Participation on a data safety monitoring board or advisory board: ALX Oncology, Metafines. Shukui Qin: None to disclose. Kensei Yamaguchi: Research grants: Taiho Pharmaceutical; Speakers bureau: Daiichi Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., Eli Lilly Japan K.K., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Merck Biopharm Co., Ltd. In-Ho Kim: None to disclose. Anwaar Saeed: Research grants (to institution): AstraZeneca, BMS, Merck, Clovis, Exelixis, Actuate Therapeutics, Incyte Corporation, Daiichi Sankyo, Five Prime Therapeutics, Amgen, Innovent Biologics, Dragonfly Therapeutics, KAHR Medical, and BioNTech; Advisory board fees: AstraZeneca, BMS, Exelixis, Pfizer, and Daiichi Sankyo. Sang Cheul Oh: None to disclose. Jin Li: Research grants: Roche; Speakers bureau: Eli Lilly, AstraZeneca. Haci Mehmet Turk: None to disclose. Alexandra Teixeira: Consulting fees: Gilead, Daiichi; Non-remunerative positions of influence: Member of SPO (Sociedade Portuguesa de Oncologia). Erika Hitre: None to disclose. Adrian A. Udrea: Honoraria: AstraZeneca, BMS, Lilly, Novartis, Sandoz, Teva; Consulting/Advisory role: Amgen, BMS, Teva; Travel, accommodations, expenses: Astellas Pharma, Teva. Giovanni Gerardo Cardellino: None to disclose. Raquel Guardeño Sanchez: Consulting fees: Ipsen, Novartis. Anita Zahlten-Kümeli: Employee and stockholder of Amgen Inc. Kate Taylor: Employee and stockholder of Amgen Inc. Peter C. Enzinger: Consultant: ALX Oncology, Amgen, Arcus Biosciences, Astellas, AstraZeneca, Boehringer Ingelheim, Blueprint Medicines, BMS, Chimeric Therapeutics, Celgene, Coherus, Daiichi Sankyo, IDEAYA, Istari, Legend, Lilly, Loxo, Merck Sharp & Dohme, Novartis, Ono, Servier, Taiho, Takeda, Turning Point Therapeutics, Xencor, Zymeworks.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

