Camptothesome-based combination nanotherapeutic regimen for improved colorectal cancer immunochemotherapy
- PMID: 38309054
- PMCID: PMC10922823
- DOI: 10.1016/j.biomaterials.2024.122477
Camptothesome-based combination nanotherapeutic regimen for improved colorectal cancer immunochemotherapy
Abstract
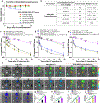
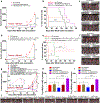
Camptothesome is a sphingomyelin-conjugated camptothecin (SM-CSS-CPT) nanovesicle that fortified the therapeutic delivery of CPT in diverse cancer types. To mitigate the Camptothesome-induced IDO1 negative feedback mechanism, we had co-encapsulated, indoximod (IND, IDO1 inhibitor) into Camptothesome using doxorubicin-derived IND (DOX-IND). To maximize the therapeutic potential of DOX-IND/Camptothesome, herein, we first dissected the synergistic drug ratio (DOX-IND/SM-CSS-CPT) via systematical in vitro screening. DOX-IND/Camptothesome with optimal drug ratio synchronized in vivo drug delivery with significantly higher tumor uptake compared to free drugs. This optimum DOX-IND/Camptothesome outperformed the combination of Camptothesome, Doxil and IND or other IDO1 inhibitors (BMS-986205 or epacadostat) in treating mice bearing late-stage MC38 tumors, and combination with immune checkpoint blockade (ICB) enabled it to eradicate 60 % of large tumors. Further, this optimized co-delivery Camptothesome beat Folfox and Folfiri, two first-line combination chemotherapies for colorectal cancer in antitumor efficacy and exhibited no side effects as compared to the severe systemic toxicities associated with Folfox and Folfiri. Finally, we demonstrated that the synergistic DOX-IND/Camptothesome was superior to the combined use of Onivyde + Doxil + IND in curbing the advanced orthotopic CT26-Luc tumors and eliminated 40 % tumors with complete metastasis remission when cooperated with ICB, eliciting stronger anti-CRC immune responses and greater reversal of immunosuppression. These results corroborated that with precise optimal synergistic drug ratio, the therapeutic potential of DOX-IND/Camptothesome can be fully unleased, which warrants further clinical investigation to benefit the cancer patients.
Keywords: Camptothesome; Colorectal cancer; Immunochemotherapy; Indoleamine 2,3-dioxygenase; Indoximod.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jianqin Lu has patent Immunogenic Nanovesicles for Cancer Immunotherapy pending to New International Patent Application No. PCT/US2021/060642.
Figures





Similar articles
-
Enhanced delivery of camptothecin to colorectal carcinoma using a tumor-penetrating peptide targeting p32.Acta Biomater. 2025 Jun 15;200:629-640. doi: 10.1016/j.actbio.2025.05.036. Epub 2025 May 14. Acta Biomater. 2025. PMID: 40379119 Free PMC article.
-
Multi-Focused Acoustic Radiation Force Impulse Modulation of Murine Hepatic Xenografts Enhances Nanoscale DOX@Lip Delivery and Therapeutic Effect.Int J Nanomedicine. 2025 Jun 12;20:7359-7373. doi: 10.2147/IJN.S522247. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40529535 Free PMC article.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574
-
Pegylated liposomal doxorubicin for first-line treatment of epithelial ovarian cancer.Cochrane Database Syst Rev. 2013 Oct 21;2013(10):CD010482. doi: 10.1002/14651858.CD010482.pub2. Cochrane Database Syst Rev. 2013. PMID: 24142521 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Enhanced delivery of camptothecin to colorectal carcinoma using a tumor-penetrating peptide targeting p32.Acta Biomater. 2025 Jun 15;200:629-640. doi: 10.1016/j.actbio.2025.05.036. Epub 2025 May 14. Acta Biomater. 2025. PMID: 40379119 Free PMC article.
-
Cholesterol-modified sphingomyelin chimeric lipid bilayer for improved therapeutic delivery.Nat Commun. 2024 Mar 7;15(1):2073. doi: 10.1038/s41467-024-46331-7. Nat Commun. 2024. PMID: 38453918 Free PMC article.
-
Multifaceted Applications of Nanomaterials in Colorectal Cancer Management: Screening, Diagnostics, and Therapeutics.Int J Nanomedicine. 2025 Jun 10;20:7271-7294. doi: 10.2147/IJN.S520616. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40520059 Free PMC article. Review.
-
Liposome-Enabled Nanomaterials for Muscle Regeneration.Small Methods. 2025 Aug;9(8):e2402154. doi: 10.1002/smtd.202402154. Epub 2025 Feb 18. Small Methods. 2025. PMID: 39967365 Review.
References
-
- Zhao H, Lee C, Sai P, Choe YH, Boro M, Pendri A, Guan S, Greenwald RB, 20-O-acylcamptothecin derivatives: evidence for lactone stabilization, J Org Chem 65(15) (2000) 4601–4606. - PubMed
-
- Catenacci DVT, Hochster H, Klempner SJ, Keeping Checkpoint Inhibitors in Check, JAMA Netw. Open 2(5) (2019) e192546. - PubMed
-
- Overman MJ, Ernstoff MS, Morse MA, Where We Stand With Immunotherapy in Colorectal Cancer: Deficient Mismatch Repair, Proficient Mismatch Repair, and Toxicity Management, Am. Soc. Clin. Oncol. Educ. Book 38 (2018) 239–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

