The Efficacy and Safety of Inhaled Antibiotics for the Treatment of Bronchiectasis in Adults: Updated Systematic Review and Meta-Analysis
- PMID: 38309462
- PMCID: PMC11251083
- DOI: 10.1016/j.chest.2024.01.045
The Efficacy and Safety of Inhaled Antibiotics for the Treatment of Bronchiectasis in Adults: Updated Systematic Review and Meta-Analysis
Abstract
Background: Inhaled antibiotics are recommended conditionally by international bronchiectasis guidelines for the treatment of patients with bronchiectasis, but results of individual studies are inconsistent. A previous meta-analysis demonstrated promising results regarding the efficacy and safety of inhaled antibiotics in bronchiectasis. Subsequent publications have supplemented the existing body of evidence further in this area.
Research question: To what extent do inhaled antibiotics demonstrate both efficacy and safety as a treatment option for adults with bronchiectasis?
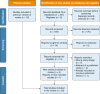
Study design and methods: Systematic review and meta-analysis of randomized controlled trials of inhaled antibiotics in adult patients with bronchiectasis. We searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, Web of Science, and ClinicalTrials.gov for eligible studies. Studies were included if they enrolled adults with bronchiectasis diagnosed by CT imaging and had a treatment duration of at least 4 weeks. The primary end point was exacerbation frequency, with additional key efficacy end points including severe exacerbations, bacterial load, symptoms, quality of life, and FEV1. Data were pooled through random-effects meta-analysis.
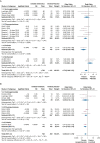
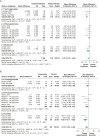
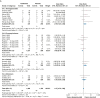
Results: Twenty studies involving 3,468 patients were included. Inhaled antibiotics were associated with reduced number of patients with exacerbations (risk ratio, 0.85; 95% CI, 0.75-0.96), a slight reduction in exacerbation frequency (rate ratio [RR], 0.78; 95% CI, 0.68-0.91), a probable reduction in the frequency of severe exacerbations (RR, 0.48; 95% CI, 0.31-0.74), and a likely slight increase in time to first exacerbation (hazard ratio, 0.80; 95% CI, 0.68-0.94). Inhaled antibiotics likely lead to a slight increase in the Quality of Life Questionnaire-Bronchiectasis respiratory symptom score (mean difference, 2.51; 95% CI, 0.44-4.31) and may reduce scores on the St. George Respiratory Questionnaire (mean difference, -3.13; 95% CI, -5.93 to -0.32). Bacterial load consistently was reduced, but FEV1 was not changed with treatment. Evidence suggests little to no difference in adverse effects between groups (OR, 0.99; 95% CI, 0.75-1.30). Antibiotic-resistant organisms likely were increased by treatment.
Interpretation: In this systematic review and meta-analysis, inhaled antibiotics resulted in a slight reduction in exacerbations, a probable reduction in severe exacerbations, and a likely slight improvement in symptoms and quality of life in adults with bronchiectasis.
Trial registry: International Prospective Register of Systematic Reviews; No.: CRD42023384694; URL: https://www.crd.york.ac.uk/prospero/.
Keywords: antibiotics; bronchiectasis; inhalation; meta-analysis; therapeutics.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial/Nonfinancial Disclosures The authors have reported to CHEST the following: H. C. reports grants or contracts from the Korean Ministry of Education Basic Science Research Program [Grant 2021R1I1A3052416]; consulting fees from Boryung Pharmaceutical Co., Ltd.; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Boryung Pharmaceutical Co., Ltd. C. S. H. reports consulting fees from 30 Technology, Aradigm, CSL Behring, Chiesi, Gilead, Grifols, GSK, Insmed, Janssen, LifeArc, Meiji, Mylan, Novartis, Pneumagen, Shionogi, Teva, Vertex, and Zambon (personal fees); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Chiesi, Insmed; and payment for expert testimony from Zambon. J. D. C. has received research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Grifols, Novartis, Insmed, and Trudell and has received consultancy or speaker fees from Antabio, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Insmed, Janssen, Novartis, Pfizer, Trudell, and Zambon. None declared (R. C.).
Figures



Similar articles
-
The efficacy and safety of inhaled antibiotics for the treatment of bronchiectasis in adults: a systematic review and meta-analysis.Lancet Respir Med. 2019 Oct;7(10):855-869. doi: 10.1016/S2213-2600(19)30185-7. Epub 2019 Aug 9. Lancet Respir Med. 2019. PMID: 31405826
-
Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis.Lancet Respir Med. 2019 Oct;7(10):845-854. doi: 10.1016/S2213-2600(19)30191-2. Epub 2019 Aug 9. Lancet Respir Med. 2019. PMID: 31405828
-
Inhaled antibiotics therapy for stable non-cystic fibrosis bronchiectasis: a meta-analysis.Ther Adv Respir Dis. 2020 Jan-Dec;14:1753466620936866. doi: 10.1177/1753466620936866. Ther Adv Respir Dis. 2020. PMID: 32615859 Free PMC article.
-
Efficacy of inhaled ciprofloxacin agents for the treatment of bronchiectasis: a systematic review and meta-analysis of randomized controlled trials.Ther Adv Respir Dis. 2019 Jan-Dec;13:1753466619875930. doi: 10.1177/1753466619875930. Ther Adv Respir Dis. 2019. PMID: 31538535 Free PMC article.
-
Efficacy and safety of long-term inhaled antibiotic for patients with noncystic fibrosis bronchiectasis: a meta-analysis.Clin Respir J. 2016 Nov;10(6):731-739. doi: 10.1111/crj.12278. Epub 2015 Mar 2. Clin Respir J. 2016. PMID: 25620629 Review.
Cited by
-
Exacerbations of bronchiectasis.Eur Respir Rev. 2024 Jul 24;33(173):240085. doi: 10.1183/16000617.0085-2024. Print 2024 Jul. Eur Respir Rev. 2024. PMID: 39048130 Free PMC article. Review.
-
Tolerance and effectiveness of inhaled antibiotics at standard or low doses in COPD patients with chronic Pseudomonas aeruginosa bronchial infection.Sci Rep. 2025 Mar 13;15(1):8773. doi: 10.1038/s41598-025-91763-w. Sci Rep. 2025. PMID: 40082508 Free PMC article.
-
Comparative efficacy and safety of inhaled antibiotics in managing chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis and bronchiectasis: a systematic review and network meta-analysis.J Thorac Dis. 2025 Mar 31;17(3):1424-1443. doi: 10.21037/jtd-24-1525. Epub 2025 Mar 27. J Thorac Dis. 2025. PMID: 40223951 Free PMC article.
-
Therapeutic Interventions for Pseudomonas Infections in Cystic Fibrosis Patients: A Review of Phase IV Trials.J Clin Med. 2024 Oct 30;13(21):6530. doi: 10.3390/jcm13216530. J Clin Med. 2024. PMID: 39518670 Free PMC article. Review.
-
Phenotypes and endotypes in bronchiectasis: a narrative review of progress toward precision medicine.J Thorac Dis. 2025 Apr 30;17(4):2640-2654. doi: 10.21037/jtd-2024-1945. Epub 2025 Apr 28. J Thorac Dis. 2025. PMID: 40400921 Free PMC article. Review.
References
-
- Harris J.K., Zemanick E.T. Microbes in bronchiectasis: the forest or the trees? Am J Respir Crit Care Med. 2013;187(10):1044–1045. - PubMed
-
- Araújo D., Shteinberg M., Aliberti S., et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J. 2018;51(2) - PubMed
-
- Finch S., McDonnell M.J., Abo-Leyah H., Aliberti S., Chalmers J.D. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc. 2015;12(11):1602–1611. - PubMed
-
- Altenburg J., de Graaff C.S., Stienstra Y., et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309(12):1251–1259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

