D-Amino acids differentially trigger an inflammatory environment in vitro
- PMID: 38310167
- PMCID: PMC10838247
- DOI: 10.1007/s00726-023-03360-8
D-Amino acids differentially trigger an inflammatory environment in vitro
Abstract
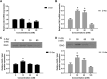
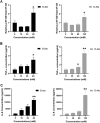
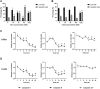
Studies in vivo have demonstrated that the accumulation of D-amino acids (D-AAs) is associated with age-related diseases and increased immune activation. However, the underlying mechanism(s) of these observations are not well defined. The metabolism of D-AAs by D-amino oxidase (DAO) produces hydrogen peroxide (H2O2), a reactive oxygen species involved in several physiological processes including immune response, cell differentiation, and proliferation. Excessive levels of H2O2 contribute to oxidative stress and eventual cell death, a characteristic of age-related pathology. Here, we explored the molecular mechanisms of D-serine (D-Ser) and D-alanine (D-Ala) in human liver cancer cells, HepG2, with a focus on the production of H2O2 the downstream secretion of pro-inflammatory cytokine and chemokine, and subsequent cell death. In HepG2 cells, we demonstrated that D-Ser decreased H2O2 production and induced concentration-dependent depolarization of mitochondrial membrane potential (MMP). This was associated with the upregulation of activated NF-кB, pro-inflammatory cytokine, TNF-α, and chemokine, IL-8 secretion, and subsequent apoptosis. Conversely, D-Ala-treated cells induced H2O2 production, and were also accompanied by the upregulation of activated NF-кB, TNF-α, and IL-8, but did not cause significant apoptosis. The present study confirms the role of both D-Ser and D-Ala in inducing inflammatory responses, but each via unique activation pathways. This response was associated with apoptotic cell death only with D-Ser. Further research is required to gain a better understanding of the mechanisms underlying D-AA-induced inflammation and its downstream consequences, especially in the context of aging given the wide detection of these entities in systemic circulation.
Keywords: D-Alanine; D-Amino acid oxidase; D-Serine; Inflammation; TNF-α.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Bardallo GR, Panisello-Roselló A, Sanchez-Nuno S, Alva N, Roselló-Catafau J, Carbonell T (2022) Nrf2 and oxidative stress in liver ischemia/reperfusion injury. FEBS J 289(18):5463–5479. 10.1111/febs.16336 - PubMed
-
- Brandish PE, Chiu C-S, Schneeweis J, Brandon NJ, Leech CL, Kornienko O, Scolnick EM, Strulovici B, Zheng W (2006) A cell-based ultra-high-throughput screening assay for identifying inhibitors of d-amino acid oxidase. J Biomol Screen 11(5):481–487. 10.1177/1087057106288181 - PubMed
-
- Buccitelli C, Selbach M (2020) mRNAs, proteins and the emerging principles of gene expression control. Nat Rev Genet 21(10):10. 10.1038/s41576-020-0258-4 - PubMed
-
- Chandel NS, Trzyna WC, McClintock DS, Schumacker PT (2000) Role of oxidants in NF-κB activation and TNF-α gene transcription induced by hypoxia and endotoxin. J Immunol 165(2):1013–1021. 10.4049/jimmunol.165.2.1013 - PubMed
-
- Chervyakov AV, Gulyaeva N, Zakharova M (2011) d-Amino acids in normal ageing and pathogenesis of neurodegenerative diseases. Neurochem J 8(2):113–129. 10.1134/S1819712411020036
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

