Wnt/β-catenin signalling activates IMPDH2-mediated purine metabolism to facilitate oxaliplatin resistance by inhibiting caspase-dependent apoptosis in colorectal cancer
- PMID: 38310229
- PMCID: PMC10838440
- DOI: 10.1186/s12967-024-04934-0
Wnt/β-catenin signalling activates IMPDH2-mediated purine metabolism to facilitate oxaliplatin resistance by inhibiting caspase-dependent apoptosis in colorectal cancer
Abstract
Background: Oxaliplatin resistance usually leads to therapeutic failure and poor prognosis in colorectal cancer (CRC), while the underlying mechanisms are not yet fully understood. Metabolic reprogramming is strongly linked to drug resistance, however, the role and mechanism of metabolic reprogramming in oxaliplatin resistance remain unclear. Here, we aim to explore the functions and mechanisms of purine metabolism on the oxaliplatin-induced apoptosis of CRC.
Methods: An oxaliplatin-resistant CRC cell line was generated, and untargeted metabolomics analysis was conducted. The inosine 5'-monophosphate dehydrogenase type II (IMPDH2) expression in CRC cell lines was determined by quantitative real-time polymerase chain reaction (qPCR) and western blotting analysis. The effects of IMPDH2 overexpression, knockdown and pharmacological inhibition on oxaliplatin resistance in CRC were assessed by flow cytometry analysis of cell apoptosis in vivo and in vitro.
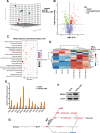
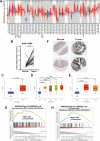
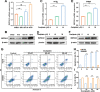
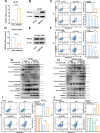
Results: Metabolic analysis revealed that the levels of purine metabolites, especially guanosine monophosphate (GMP), were markedly elevated in oxaliplatin-resistant CRC cells. The accumulation of purine metabolites mainly arose from the upregulation of IMPDH2 expression. Gene set enrichment analysis (GSEA) indicated high IMPDH2 expression in CRC correlates with PURINE_METABOLISM and MULTIPLE-DRUG-RESISTANCE pathways. CRC cells with higher IMPDH2 expression were more resistant to oxaliplatin-induced apoptosis. Overexpression of IMPDH2 in CRC cells resulted in reduced cell death upon treatment with oxaliplatin, whereas knockdown of IMPDH2 led to increased sensitivity to oxaliplatin through influencing the activation of the Caspase 7/8/9 and PARP1 proteins on cell apoptosis. Targeted inhibition of IMPDH2 by mycophenolic acid (MPA) or mycophenolate mofetil (MMF) enhanced cell apoptosis in vitro and decreased in vivo tumour burden when combined with oxaliplatin treatment. Mechanistically, the Wnt/β-catenin signalling was hyperactivated in oxaliplatin-resistant CRC cells, and a reciprocal positive regulatory mechanism existed between Wnt/β-catenin and IMPDH2. Blocking the Wnt/β-catenin pathway could resensitize resistant cells to oxaliplatin, which could be restored by the addition of GMP.
Conclusions: IMPDH2 is a predictive biomarker and therapeutic target for oxaliplatin resistance in CRC.
Keywords: Apoptosis; Colorectal cancer; IMPDH2; Oxaliplatin resistance; Purine metabolism; β‑catenin.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no competing interests and approve the manuscript for publication.
Figures


Similar articles
-
IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways.J Exp Clin Cancer Res. 2018 Dec 5;37(1):304. doi: 10.1186/s13046-018-0980-3. J Exp Clin Cancer Res. 2018. PMID: 30518405 Free PMC article.
-
Mitotic Arrest Deficient 2 Like 1 Contributes to Colorectal Cancer Cell Migration, Invasion, and Oxaliplatin Resistance Through the Wnt/β-Catenin Pathway.Chem Biol Drug Des. 2024 Nov;104(5):e70012. doi: 10.1111/cbdd.70012. Chem Biol Drug Des. 2024. PMID: 39487106
-
Huaier promotes sensitivity of colorectal cancer to oxaliplatin by inhibiting METTL3 to regulate the Wnt/β‑catenin signaling pathway.Oncol Rep. 2025 Jan;53(1):7. doi: 10.3892/or.2024.8840. Epub 2024 Nov 8. Oncol Rep. 2025. PMID: 39513580 Free PMC article.
-
The Role of microRNAs in Regulating Cancer Cell Response to Oxaliplatin-Containing Regimens.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231206003. doi: 10.1177/15330338231206003. Technol Cancer Res Treat. 2023. PMID: 37849311 Free PMC article. Review.
-
A Model of Butyrate Activity and Resistance in CRC.J Cell Mol Med. 2025 Jun;29(11):e70656. doi: 10.1111/jcmm.70656. J Cell Mol Med. 2025. PMID: 40506777 Free PMC article. Review.
Cited by
-
Fecal Microbiota Transplantation Activity of Floccularia luteovirens Polysaccharides and Their Protective Effect on Cyclophosphamide-Induced Immunosuppression and Intestinal Injury in Mice.Foods. 2024 Nov 30;13(23):3881. doi: 10.3390/foods13233881. Foods. 2024. PMID: 39682952 Free PMC article.
-
Distinctive chromosomal, mutational and transcriptional profiling in colon versus rectal cancers.J Transl Med. 2025 Aug 6;23(1):869. doi: 10.1186/s12967-025-06908-2. J Transl Med. 2025. PMID: 40770356 Free PMC article.
-
Identification and predictive machine learning models construction of gut microbiota associated with lymph node metastasis in colorectal cancer.mSystems. 2025 Aug 19;10(8):e0033925. doi: 10.1128/msystems.00339-25. Epub 2025 Jul 8. mSystems. 2025. PMID: 40626716 Free PMC article.
-
IMPDH2's Central Role in Cellular Growth and Diseases: A Potential Therapeutic Target.Cell Prolif. 2025 Jun;58(6):e70031. doi: 10.1111/cpr.70031. Epub 2025 Apr 19. Cell Prolif. 2025. PMID: 40251939 Free PMC article. Review.
-
Wnt signaling pathways in biology and disease: mechanisms and therapeutic advances.Signal Transduct Target Ther. 2025 Apr 4;10(1):106. doi: 10.1038/s41392-025-02142-w. Signal Transduct Target Ther. 2025. PMID: 40180907 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

