Converting between the International Prostate Symptom Score (IPSS) and the Expanded Prostate Cancer Index Composite (EPIC) urinary subscales: modeling and external validation
- PMID: 38310268
- PMCID: PMC10837947
- DOI: 10.1186/s12894-024-01421-y
Converting between the International Prostate Symptom Score (IPSS) and the Expanded Prostate Cancer Index Composite (EPIC) urinary subscales: modeling and external validation
Abstract
Background: Prostate-related quality of life can be assessed with a variety of different questionnaires. The 50-item Expanded Prostate Cancer Index Composite (EPIC) and the International Prostate Symptom Score (IPSS) are two widely used options. The goal of this study was, therefore, to develop and validate a model that is able to convert between the EPIC and the IPSS to enable comparisons across different studies.
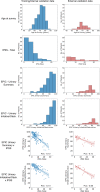
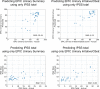
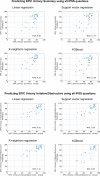
Methods: Three hundred forty-seven consecutive patients who had previously received radiotherapy and surgery for prostate cancer at two institutions in Switzerland and Germany were contacted via mail and instructed to complete both questionnaires. The Swiss cohort was used to train and internally validate different machine learning models using fourfold cross-validation. The German cohort was used for external validation.
Results: Converting between the EPIC Urinary Irritative/Obstructive subscale and the IPSS using linear regressions resulted in mean absolute errors (MAEs) of 3.88 and 6.12, which is below the respective previously published minimal important differences (MIDs) of 5.2 and 10 points. Converting between the EPIC Urinary Summary and the IPSS was less accurate with MAEs of 5.13 and 10.45, similar to the MIDs. More complex model architectures did not result in improved performance in this study. The study was limited to the German versions of the respective questionnaires.
Conclusions: Linear regressions can be used to convert between the IPSS and the EPIC Urinary subscales. While the equations obtained in this study can be used to compare results across clinical trials, they should not be used to inform clinical decision-making in individual patients.
Trial registration: This study was retrospectively registered on clinicaltrials.gov on January 14th, 2022, under the registration number NCT05192876.
Keywords: EPIC; IPSS; Prostate; Quality of life.
© 2024. The Author(s).
Conflict of interest statement
P.W. has a patent application titled ‘Method for detection of neurological abnormalities’ outside of the submitted work. The remaining authors declare no conflict of interest.
Figures

Similar articles
-
Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer.Urology. 2000 Dec 20;56(6):899-905. doi: 10.1016/s0090-4295(00)00858-x. Urology. 2000. PMID: 11113727
-
Cross validation of the prostate cancer radiotherapy late toxicity (PCRT) questionnaire with the expanded prostate cancer index composite (EPIC) instrument.Can J Urol. 2011 Aug;18(4):5802-10. Can J Urol. 2011. PMID: 21854712
-
Patient-reported Quality of Life After SBRT, LDR, and HDR Brachytherapy for Prostate Cancer: A Comparison of Outcomes.Am J Clin Oncol. 2021 Apr 1;44(4):131-136. doi: 10.1097/COC.0000000000000796. Am J Clin Oncol. 2021. PMID: 33577175
-
Patient-reported outcome (PRO) questionnaires for men who have radical surgery for prostate cancer: a conceptual review of existing instruments.BJU Int. 2017 Oct;120(4):468-481. doi: 10.1111/bju.13896. Epub 2017 May 30. BJU Int. 2017. PMID: 28437031 Review.
-
How international is the International Prostate Symptom Score? A literature review of validated translations of the IPSS, the most widely used self-administered patient questionnaire for male lower urinary tract symptoms.Low Urin Tract Symptoms. 2022 Mar;14(2):92-101. doi: 10.1111/luts.12415. Epub 2021 Nov 3. Low Urin Tract Symptoms. 2022. PMID: 34734477 Review.
Cited by
-
Rezūm water vapor therapy vs. thulium laser enucleation for the treatment of benign prostatic hyperplasia in patients with large prostates: a multicenter prospective comparative study.Prostate Cancer Prostatic Dis. 2025 Apr 14. doi: 10.1038/s41391-025-00971-y. Online ahead of print. Prostate Cancer Prostatic Dis. 2025. PMID: 40229454

