The Sjögren's Working Group: The 2023 OMERACT meeting and provisional domain generation
- PMID: 38310657
- PMCID: PMC10954392
- DOI: 10.1016/j.semarthrit.2024.152378
The Sjögren's Working Group: The 2023 OMERACT meeting and provisional domain generation
Abstract
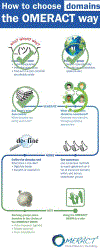
Sjögren's disease (SjD) is a systemic autoimmune exocrinopathy with key features of dryness, pain, and fatigue. SjD can affect any organ system with a variety of presentations across individuals. This heterogeneity is one of the major barriers for developing effective disease modifying treatments. Defining core disease domains comprising both specific clinical features and incorporating the patient experience is a critical first step to define this complex disease. The OMERACT SjD Working Group held its first international collaborative hybrid meeting in 2023, applying the OMERACT 2.2 filter toward identification of core domains. We accomplished our first goal, a scoping literature review that was presented at the Special Interest Group held in May 2023. Building on the domains identified in the scoping review, we uniquely deployed multidisciplinary experts as part of our collaborative team to generate a provisional domain list that captures SjD heterogeneity.
Keywords: Artificial intelligence; BIBOT; OMERACT; Review; Scoping literature review; Sjogren's disease.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest Valerie Devauchelle reports receinving funds for consulting to Novartis, Abbvie, Fresenius Kabi. Divi Cornec declares no personal financial competing interests and received research funding from Novartis and GSK. Benjamin A. Fisher has undertaken consultancy for Novartis, BMS, Servier, Galapagos, Roche, UCB, Sanofi and Janssen, and received grant/research support from Janssen, Celgene, Galapagos, Servier. Alberta Hoi reports receiving research funding from AstraZeneca, Bristol-Myers Squibb, Novartis, Janssen. Chiara Baldini reports receiving funds for consulting to GSK, Novartis and Horizon, honoraria for educational events from GSK and Sanofi, support for attending meetings from Abbvie and Bristol-Myers Squibb. WF Ng has consulted for Novartis, GlaxoSmithKline, Abbvie, BMS, Sanofi, MedImmune, Resolves Therapeutics, Janssen and UCB. Simon Bowman receiving funds for consulting from Bristol-Myers Squibb, Iqvia, Janssen, Kiniksa, Novartis, Otsuka-Visterra. His-salary is part funded by the Birmingham Biomedical Research Centre, Birmingham, UK. Karina Torralba reports receiving funds for consulting to Horizon, AstraZeneca, Janssen; for contracted research work with Bioclinica; for clinical trial funding from Novartis, AstraZeneca, GlaxoSmithKline, Amgen. Athena Papas declares grant funding from Novartis and Horizon; advisory board for Novartis. Ionut Pintilie reports receiving funds for consulting to Abbvie, Novartis, Pfizer, Sandoz, Ewopharma, KRKA, Stada, Boehringer Ingelheim, MagnaPharm, MSD. Xavier Mariette declares consulting fee from Astra Zeneca, BMS, Galapagos, GSK, Novartis, and Pfizer. Maria Antonietta D'Agostino, MD, PhD Speakers, or consultant fees from Amgen, Abbvie, BMS, Novartis, Galapagos, UCB, Pfizer, Lily, Janssen. Alan Baer reports receiving funds for consulting to Bristol-Myers Squibb and iCell Gene Therapeutics. Blake M. Warner declares research funding and material transfer agreements with Pfizer, Inc., and Mitobridge, Inc. Soumya D. Chakravarty is an employee of Janssen Scientific Affairs, LLC, and owns stock or stock options in Johnson & Johnson, of which Janssen Scientific Affairs, LLC is a wholly owned subsidiary. Nevsun Inanc reports claims to have received speakers fee from Novartis, Abbvie, Pfizer, UCB, Eli-Lilly and consultancy fee from Abbvie, UCB, Eli-Lilly. Vibeke Strand reports being a founding member of the executive committee of Outcome Measures in Rheumatology (OMERACT) [1992 – present], an international consensus organization that develops and validates outcome measures in rheumatology randomized controlled trials and longitudinal observational studies and has received arms-length funding from as many as 36 sponsors. Md Yuzaiful Md Yusof has received speaker fees from Roche and Novartis and consultancy fees from Aurinia Pharmaceuticals and UCB. Suzanne Arends declares consultancy fees from Argenx and Novartis. Anas Alexis Benyoussef is a consultant for Horus Pharma and Quantel Medical. Sharmila Masli is a consultant for Stellular Bio Inc. and Proteris Biotech. Maureen Rischmueller has undertaken consultancy/speaker engagements for AbbVie, Boehringer Ingelheim, Janssen Global Services, Novartis, Pfizer and Sandoz, and received grant/research support from AbbVie, Amgen, AstraZeneca, BMS, GSK, Janssen, Lilly, Novartis, Pfizer, Servier and UCB. Sara McCoy reports receiving funds for consulting to Bristol-Myers Squibb, Horizon, Novartis, Kiniksa, Targe RWE, Otsuka, Visterra, and iCell. Her time is supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant 1KL2TR002374 and NIH/NIDCR R03DE031340. Raphaele Seror reports receiving funds for consulting to Bristol-Myers Squibb, Novartis, GSK, Janssen, Amgen. Hendrika Bootsma declares consultancy fees from Argenx, Novartis, BMS, AztraZeneca, Galapagos.Independent grants from AstraZeneca, Novartis, BMS.
Figures

References
-
- Miyamoto ST, Valim V, Fisher BA. Health-related quality of life and costs in Sjögren’s syndrome. Rheumatology (Oxford). 2019. - PubMed
-
- Dias LH, Miyamoto ST, Giovelli RA, de Magalhães CIM, Valim V. Pain and fatigue are predictors of quality of life in primary Sjögren’s syndrome. Adv Rheumatol. 2021;61(1):28. - PubMed
-
- Bowman SJ, St Pierre Y, Sutcliffe N, Isenberg DA, Goldblatt F, Price E, et al. Estimating indirect costs in primary Sjogren’s syndrome. J Rheumatol. 2010;37(5):1010–5. - PubMed
-
- Boers M, Beaton DE, Shea BJ, Maxwell LJ, Bartlett SJ, Bingham CO, 3rd, et al. OMERACT Filter 2.1: Elaboration of the Conceptual Framework for Outcome Measurement in Health Intervention Studies. J Rheumatol. 2019;46(8):1021–7. - PubMed
-
- Maxwell LJ, Beaton DE, Boers M, D’Agostino MA, Conaghan PG, Grosskleg S, et al. The evolution of instrument selection for inclusion in core outcome sets at OMERACT: Filter 2.2. Semin Arthritis Rheum. 2021;51(6):1320–30. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

