Proof-of-concept study of the caninized anti-canine programmed death 1 antibody in dogs with advanced non-oral malignant melanoma solid tumors
- PMID: 38311328
- PMCID: PMC10839171
- DOI: 10.4142/jvs.23144
Proof-of-concept study of the caninized anti-canine programmed death 1 antibody in dogs with advanced non-oral malignant melanoma solid tumors
Abstract
Background: The anti-programmed death 1 (PD-1) antibody has led to durable clinical responses in a wide variety of human tumors. We have previously developed the caninized anti-canine PD-1 antibody (ca-4F12-E6) and evaluated its therapeutic properties in dogs with advance-staged oral malignant melanoma (OMM), however, their therapeutic effects on other types of canine tumors remain unclear.
Objective: The present clinical study was carried out to evaluate the safety profile and clinical efficacy of ca-4F12-E6 in dogs with advanced solid tumors except for OMM.
Methods: Thirty-eight dogs with non-OMM solid tumors were enrolled prospectively and treated with ca-4F12-E6 at 3 mg/kg every 2 weeks of each 10-week treatment cycle. Adverse events (AEs) and treatment efficacy were graded based on the criteria established by the Veterinary Cooperative Oncology Group.
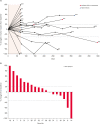
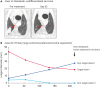
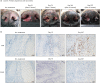
Results: One dog was withdrawn, and thirty-seven dogs were evaluated for the safety and efficacy of ca-4F12-E6. Treatment-related AEs of any grade occurred in 13 out of 37 cases (35.1%). Two dogs with sterile nodular panniculitis and one with myasthenia gravis and hypothyroidism were suspected of immune-related AEs. In 30 out of 37 dogs that had target tumor lesions, the overall response and clinical benefit rates were 6.9% and 27.6%, respectively. The median progression-free survival and overall survival time were 70 days and 215 days, respectively.
Conclusions: The present study demonstrated that ca-4F12-E6 was well-tolerated in non-OMM dogs, with a small number of cases showing objective responses. This provides evidence supporting large-scale clinical trials of anti-PD-1 antibody therapy in dogs.
Keywords: Dog; immune checkpoint inhibitor; immunotherapy; monoclonal antibody; tumor.
© 2024 The Korean Society of Veterinary Science.
Conflict of interest statement
Takuya Mizuno received research funding from Nippon Zenyaku Kogyo Co., Ltd. The remaining authors declare no conflicts of interest.
Figures
References
-
- Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–3758. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

