Extracellular vesicles as tools and targets in therapy for diseases
- PMID: 38311623
- PMCID: PMC10838959
- DOI: 10.1038/s41392-024-01735-1
Extracellular vesicles as tools and targets in therapy for diseases
Abstract
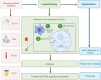
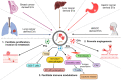
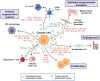
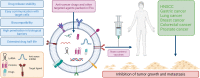
Extracellular vesicles (EVs) are nano-sized, membranous structures secreted into the extracellular space. They exhibit diverse sizes, contents, and surface markers and are ubiquitously released from cells under normal and pathological conditions. Human serum is a rich source of these EVs, though their isolation from serum proteins and non-EV lipid particles poses challenges. These vesicles transport various cellular components such as proteins, mRNAs, miRNAs, DNA, and lipids across distances, influencing numerous physiological and pathological events, including those within the tumor microenvironment (TME). Their pivotal roles in cellular communication make EVs promising candidates for therapeutic agents, drug delivery systems, and disease biomarkers. Especially in cancer diagnostics, EV detection can pave the way for early identification and offers potential as diagnostic biomarkers. Moreover, various EV subtypes are emerging as targeted drug delivery tools, highlighting their potential clinical significance. The need for non-invasive biomarkers to monitor biological processes for diagnostic and therapeutic purposes remains unfulfilled. Tapping into the unique composition of EVs could unlock advanced diagnostic and therapeutic avenues in the future. In this review, we discuss in detail the roles of EVs across various conditions, including cancers (encompassing head and neck, lung, gastric, breast, and hepatocellular carcinoma), neurodegenerative disorders, diabetes, viral infections, autoimmune and renal diseases, emphasizing the potential advancements in molecular diagnostics and drug delivery.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

